Cartilage Formation In Vivo Using High Concentration Collagen-Based Bioink with MSC and Decellularized ECM Granules
- PMID: 35269850
- PMCID: PMC8910854
- DOI: 10.3390/ijms23052703
Cartilage Formation In Vivo Using High Concentration Collagen-Based Bioink with MSC and Decellularized ECM Granules
Abstract
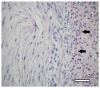
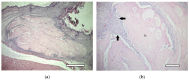
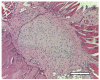
The aim of this study was to verify the applicability of high-concentration collagen-based bioink with MSC (ADSC) and decellularized ECM granules for the formation of cartilage tissue de novo after subcutaneous implantation of the scaffolds in rats. The printability of the bioink (4% collagen, 2.5% decellularized ECM granules, derived via 280 μm sieve) was shown. Three collagen-based compositions were studied: (1) with ECM; (2) with MSC; (3) with ECM and MSC. It has been established that decellularized ECM granules are able to stimulate chondrogenesis both in cell-free and MSC-laden scaffolds. Undesirable effects have been identified: bone formation as well as cartilage formation outside of the scaffold area. The key perspectives and limitations of ECM granules (powder) application have been discussed.
Keywords: ECM; MSC; bioink; bioprinting; cartilage; collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


















References
-
- Arguchinskaya N.V., Beketov E.E., Isaeva E.V., Sergeeva N.S., Shegay P.V., Ivanov S.A., Kaprin A.D. Materials for creating tissue-engineered constructs using 3D bioprinting: Cartilaginous and soft tissue restoration. Russ. J. Transpl. Artif. Organs. 2021;23:60–74. doi: 10.15825/1995-1191-2021-1-60-74. - DOI
-
- Beketov E.E., Isaeva E.V., Shegai P.V., Ivanov S.A., Kaprin A.D. Current state of tissue engineering for cartilage regeneration. Genes Cells. 2019;14:12–20. doi: 10.23868/201906013. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

