Low-Dose Delta-9-Tetrahydrocannabinol as Beneficial Treatment for Aged APP/PS1 Mice
- PMID: 35269905
- PMCID: PMC8910894
- DOI: 10.3390/ijms23052757
Low-Dose Delta-9-Tetrahydrocannabinol as Beneficial Treatment for Aged APP/PS1 Mice
Abstract
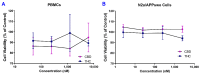
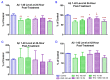
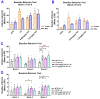
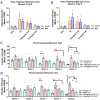
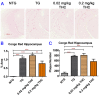
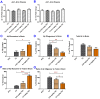
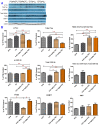
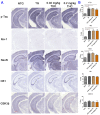
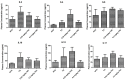
Studies on the effective and safe therapeutic dosage of delta-9-tetrahydrocannabinol (THC) for the treatment of Alzheimer's disease (AD) have been sparse due to the concern about THC's psychotropic activity. The present study focused on demonstrating the beneficial effect of low-dose THC treatment in preclinical AD models. The effect of THC on amyloid-β (Aβ) production was examined in N2a/AβPPswe cells. An in vivo study was conducted in aged APP/PS1 transgenic mice that received an intraperitoneal injection of THC at 0.02 and 0.2 mg/kg every other day for three months. The in vitro study showed that THC inhibited Aβ aggregation within a safe dose range. Results of the radial arm water maze (RAWM) test demonstrated that treatment with 0.02 and 0.2 mg/kg of THC for three months significantly improved the spatial learning performance of aged APP/PS1 mice in a dose-dependent manner. Results of protein analyses revealed that low-dose THC treatment significantly decreased the expression of Aβ oligomers, phospho-tau and total tau, and increased the expression of Aβ monomers and phospho-GSK-3β (Ser9) in the THC-treated brain tissues. In conclusion, treatment with THC at 0.2 and 0.02 mg/kg improved the spatial learning of aged APP/PS1 mice, suggesting low-dose THC is a safe and effective treatment for AD.
Keywords: APP/PS1 transgenic mice; Alzheimer’s disease; amyloid-β; cannabidiol; delta-9-tetrahydrocannabinol; radial arm water maze test.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study.
Figures
Similar articles
-
Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer's Disease.Biomolecules. 2023 Jan 25;13(2):232. doi: 10.3390/biom13020232. Biomolecules. 2023. PMID: 36830601 Free PMC article.
-
The potential therapeutic effects of THC on Alzheimer's disease.J Alzheimers Dis. 2014;42(3):973-84. doi: 10.3233/JAD-140093. J Alzheimers Dis. 2014. PMID: 25024327
-
GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice.Curr Alzheimer Res. 2009 Apr;6(2):118-31. doi: 10.2174/156720509787602942. Curr Alzheimer Res. 2009. PMID: 19355846
-
CART modulates beta-amyloid metabolism-associated enzymes and attenuates memory deficits in APP/PS1 mice.Neurol Res. 2017 Oct;39(10):885-894. doi: 10.1080/01616412.2017.1348689. Epub 2017 Jul 25. Neurol Res. 2017. PMID: 28743230
-
The novel multi-target iron chelating-radical scavenging compound M30 possesses beneficial effects on major hallmarks of Alzheimer's disease.Antioxid Redox Signal. 2012 Sep 15;17(6):860-77. doi: 10.1089/ars.2011.4279. Epub 2012 Apr 17. Antioxid Redox Signal. 2012. PMID: 22360429
Cited by
-
Emerging Therapeutic Potential of Cannabidiol (CBD) in Neurological Disorders: A Comprehensive Review.Behav Neurol. 2023 Oct 12;2023:8825358. doi: 10.1155/2023/8825358. eCollection 2023. Behav Neurol. 2023. PMID: 37868743 Free PMC article. Review.
-
Multi-Targeting Intranasal Nanoformulation as a Therapeutic for Alzheimer's Disease.Biomolecules. 2023 Jan 25;13(2):232. doi: 10.3390/biom13020232. Biomolecules. 2023. PMID: 36830601 Free PMC article.
-
An Ultra-Low Dose of ∆9-Tetrahydrocannabinol Alleviates Alzheimer's Disease-Related Cognitive Impairments and Modulates TrkB Receptor Expression in a 5XFAD Mouse Model.Int J Mol Sci. 2022 Aug 21;23(16):9449. doi: 10.3390/ijms23169449. Int J Mol Sci. 2022. PMID: 36012711 Free PMC article.
-
Psychoactive substances: novel molecular insights and therapeutic potential for Alzheimer's disease.Transl Neurodegener. 2025 Jul 25;14(1):38. doi: 10.1186/s40035-025-00498-1. Transl Neurodegener. 2025. PMID: 40713680 Free PMC article. Review.
-
Δ8-THC Protects against Amyloid Beta Toxicity Modulating ER Stress In Vitro: A Transcriptomic Analysis.Int J Mol Sci. 2023 Apr 2;24(7):6598. doi: 10.3390/ijms24076598. Int J Mol Sci. 2023. PMID: 37047608 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

