Coenzyme A Restriction as a Factor Underlying Pre-Eclampsia with Polycystic Ovary Syndrome as a Risk Factor
- PMID: 35269927
- PMCID: PMC8911031
- DOI: 10.3390/ijms23052785
Coenzyme A Restriction as a Factor Underlying Pre-Eclampsia with Polycystic Ovary Syndrome as a Risk Factor
Abstract
Pre-eclampsia is the most common pregnancy complication affecting 1 in 20 pregnancies, characterized by high blood pressure and signs of organ damage, most often to the liver and kidneys. Metabolic network analysis of published lipidomic data points to a shortage of Coenzyme A (CoA). Gene expression profile data reveal alterations to many areas of metabolism and, crucially, to conflicting cellular regulatory mechanisms arising from the overproduction of signalling lipids driven by CoA limitation. Adverse feedback loops appear, forming sphingosine-1-phosphate (a cause of hypertension, hypoxia and inflammation), cytotoxic isoketovaleric acid (inducing acidosis and organ damage) and a thrombogenic lysophosphatidyl serine. These also induce mitochondrial and oxidative stress, leading to untimely apoptosis, which is possibly the cause of CoA restriction. This work provides a molecular basis for the signs of pre-eclampsia, why polycystic ovary syndrome is a risk factor and what might be done to treat and reduce the risk of disease.
Keywords: Coenzyme A; adverse antenatal conditions; genome-wide association study; metabolomics; physiological dysregulation; placenta; polycystic ovary syndrome; pre-eclampsia; systems pathology; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
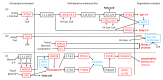
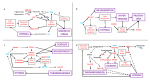
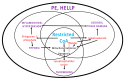
Similar articles
-
Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology.Hum Reprod Update. 2014 Mar-Apr;20(2):293-307. doi: 10.1093/humupd/dmt054. Epub 2013 Oct 16. Hum Reprod Update. 2014. PMID: 24132226 Review.
-
MicroRNAs: are they the missing link between hypoxia and pre-eclampsia?Hypertens Pregnancy. 2014 Feb;33(1):102-14. doi: 10.3109/10641955.2013.832772. Epub 2013 Dec 19. Hypertens Pregnancy. 2014. PMID: 24354525 Review.
-
A Narrative Review of Placental Contribution to Adverse Pregnancy Outcomes in Women With Polycystic Ovary Syndrome.J Clin Endocrinol Metab. 2019 Nov 1;104(11):5299-5315. doi: 10.1210/jc.2019-00383. J Clin Endocrinol Metab. 2019. PMID: 31393571 Free PMC article. Review.
-
Activin signalling and pre-eclampsia: from genetic risk to pre-symptomatic biomarker.Cytokine. 2015 Feb;71(2):360-5. doi: 10.1016/j.cyto.2014.11.017. Epub 2014 Dec 13. Cytokine. 2015. PMID: 25510903 Review.
-
Lifestyle and pregnancy complications in polycystic ovary syndrome: The SCOPE cohort study.Clin Endocrinol (Oxf). 2019 Jun;90(6):814-821. doi: 10.1111/cen.13954. Epub 2019 Apr 1. Clin Endocrinol (Oxf). 2019. PMID: 30801750
Cited by
-
Clinical study on the difference in intestinal microecology between patients with preeclampsia and pregnant women at different stages of pregnancy.Acta Biochim Pol. 2024 Mar 20;71:12020. doi: 10.3389/abp.2024.12020. eCollection 2024. Acta Biochim Pol. 2024. PMID: 38721310 Free PMC article.
-
Pregnancy Metabolic Adaptation and Changes in Placental Metabolism in Preeclampsia.Geburtshilfe Frauenheilkd. 2024 Sep 19;84(11):1033-1042. doi: 10.1055/a-2403-4855. eCollection 2024 Nov. Geburtshilfe Frauenheilkd. 2024. PMID: 39524034 Free PMC article.
-
Dissecting the Impact of Maternal Androgen Exposure on Developmental Programming through Targeting the Androgen Receptor.Adv Sci (Weinh). 2024 Sep;11(36):e2309429. doi: 10.1002/advs.202309429. Epub 2024 Jul 29. Adv Sci (Weinh). 2024. PMID: 39075722 Free PMC article.
-
Polycystic Ovarian Syndrome (PCOS): Does the Challenge End at Conception?Int J Environ Res Public Health. 2022 Nov 12;19(22):14914. doi: 10.3390/ijerph192214914. Int J Environ Res Public Health. 2022. PMID: 36429632 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

