Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches
- PMID: 35269969
- PMCID: PMC8910833
- DOI: 10.3390/ijms23052826
Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches
Abstract
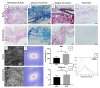
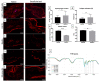
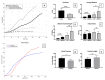
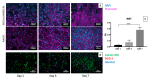
Bladder cancer (BC) is among the most common malignancies in the world and a relevant cause of cancer mortality. BC is one of the most frequent causes for bladder removal through radical cystectomy, the gold-standard treatment for localized muscle-invasive and some cases of high-risk, non-muscle-invasive bladder cancer. In order to restore urinary functionality, an autologous intestinal segment has to be used to create a urinary diversion. However, several complications are associated with bowel-tract removal, affecting patients' quality of life. The present study project aims to develop a bio-engineered material to simplify this surgical procedure, avoiding related surgical complications and improving patients' quality of life. The main novelty of such a therapeutic approach is the decellularization of a porcine small intestinal submucosa (SIS) conduit to replace the autologous intestinal segment currently used as urinary diversion after radical cystectomy, while avoiding an immune rejection. Here, we performed a preliminary evaluation of this acellular product by developing a novel decellularization process based on an environmentally friendly, mild detergent, i.e., Tergitol, to replace the recently declared toxic Triton X-100. Treatment efficacy was evaluated through histology, DNA, hydroxyproline and elastin quantification, mechanical and insufflation tests, two-photon microscopy, FTIR analysis, and cytocompatibility tests. The optimized decellularization protocol is effective in removing cells, including DNA content, from the porcine SIS, while preserving the integrity of the extracellular matrix despite an increase in stiffness. An effective sterilization protocol was found, and cytocompatibility of treated SIS was demonstrated from day 1 to day 7, during which human fibroblasts were able to increase in number and strongly organize along tissue fibres. Taken together, this in vitro study suggests that SIS is a suitable candidate for use in urinary diversions in place of autologous intestinal segments, considering the optimal results of decellularization and cell proliferation. Further efforts should be undertaken in order to improve SIS conduit patency and impermeability to realize a future viable substitute.
Keywords: biomaterial; decellularization; regenerative medicine; small intestinal submucosa; tissue engineering; urinary diversions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers.J Funct Biomater. 2022 Nov 5;13(4):222. doi: 10.3390/jfb13040222. J Funct Biomater. 2022. PMID: 36412863 Free PMC article.
-
Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering.Acta Biomater. 2014 Dec;10(12):5043-5054. doi: 10.1016/j.actbio.2014.08.024. Epub 2014 Aug 27. Acta Biomater. 2014. PMID: 25173840
-
Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes.J Funct Biomater. 2023 Mar 2;14(3):141. doi: 10.3390/jfb14030141. J Funct Biomater. 2023. PMID: 36976065 Free PMC article.
-
Diverse preparation methods for small intestinal submucosa (SIS): Decellularization, components, and structure.J Biomed Mater Res A. 2019 Mar;107(3):689-697. doi: 10.1002/jbm.a.36582. Epub 2018 Dec 9. J Biomed Mater Res A. 2019. PMID: 30468308 Review.
-
Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa.Tissue Eng Part B Rev. 2014 Feb;20(1):73-83. doi: 10.1089/ten.TEB.2013.0126. Epub 2013 Jul 25. Tissue Eng Part B Rev. 2014. PMID: 23777420 Free PMC article. Review.
Cited by
-
Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment.Bioengineering (Basel). 2024 Apr 28;11(5):436. doi: 10.3390/bioengineering11050436. Bioengineering (Basel). 2024. PMID: 38790303 Free PMC article.
-
Porcine Small Intestinal Submucosa Extracellular Matrix: A Meta-Analysis of Composition, Processing Techniques, and Biomedical Applications.Int J Mol Sci. 2025 Jun 29;26(13):6276. doi: 10.3390/ijms26136276. Int J Mol Sci. 2025. PMID: 40650053 Free PMC article. Review.
-
Small intestinal submucosa-derived extracellular matrix as a heterotopic scaffold for cardiovascular applications.Front Bioeng Biotechnol. 2022 Dec 12;10:1042434. doi: 10.3389/fbioe.2022.1042434. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36578513 Free PMC article.
-
Decellularized small intestine scaffolds: a potential xenograft for restoration of intestinal perforation.Tissue Barriers. 2024 Oct;12(4):2290940. doi: 10.1080/21688370.2023.2290940. Epub 2023 Dec 5. Tissue Barriers. 2024. PMID: 38053224 Free PMC article.
-
Development of UROGRAFT: A Bladder Acellular Matrix-Based Composite for Advanced Cystoplasty, Highlighting the Role of Graft Shape and Composition.ACS Biomater Sci Eng. 2025 Aug 11;11(8):4978-4999. doi: 10.1021/acsbiomaterials.5c00700. Epub 2025 Jul 16. ACS Biomater Sci Eng. 2025. PMID: 40667861 Free PMC article.
References
-
- Shabsigh A., Korets R., Vora K.C., Brooks C.M., Cronin A.M., Savage C., Raj G., Bochner B.H., Dalbagni G., Herr H.W., et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009;55:164–176. doi: 10.1016/j.eururo.2008.07.031. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

