Stem Cell Therapy: From Idea to Clinical Practice
- PMID: 35269990
- PMCID: PMC8911494
- DOI: 10.3390/ijms23052850
Stem Cell Therapy: From Idea to Clinical Practice
Abstract
Regenerative medicine is a new and promising mode of therapy for patients who have limited or no other options for the treatment of their illness. Due to their pleotropic therapeutic potential through the inhibition of inflammation or apoptosis, cell recruitment, stimulation of angiogenesis, and differentiation, stem cells present a novel and effective approach to several challenging human diseases. In recent years, encouraging findings in preclinical studies have paved the way for many clinical trials using stem cells for the treatment of various diseases. The translation of these new therapeutic products from the laboratory to the market is conducted under highly defined regulations and directives provided by competent regulatory authorities. This review seeks to familiarize the reader with the process of translation from an idea to clinical practice, in the context of stem cell products. We address some required guidelines for clinical trial approval, including regulations and directives presented by the Food and Drug Administration (FDA) of the United States, as well as those of the European Medicine Agency (EMA). Moreover, we review, summarize, and discuss regenerative medicine clinical trial studies registered on the Clinicaltrials.gov website.
Keywords: clinical trial; mesenchymal stem cell; regenerative medicine; stem cell therapy.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures
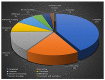
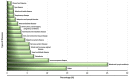
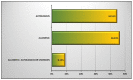
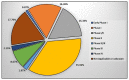
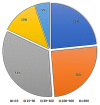
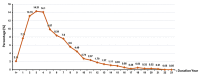
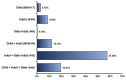
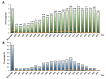
References
-
- Ulia M.P., Mantalaris S. Stem cells bioprocessing: An important milestone to move regenerative medicine research into the clinical arena. Pediatric Res. 2008;63:6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

