Isolation of Platelet-Derived Exosomes from Human Platelet-Rich Plasma: Biochemical and Morphological Characterization
- PMID: 35270001
- PMCID: PMC8911307
- DOI: 10.3390/ijms23052861
Isolation of Platelet-Derived Exosomes from Human Platelet-Rich Plasma: Biochemical and Morphological Characterization
Abstract
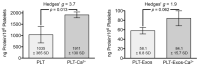
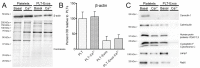
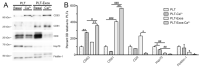
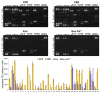
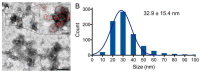
Platelet-Rich Plasma (PRP) is enriched in molecular messengers with restorative effects on altered tissue environments. Upon activation, platelets release a plethora of growth factors and cytokines, either in free form or encapsulated in exosomes, which have been proven to promote tissue repair and regeneration. Translational research on the potential of exosomes as a safe nanosystem for therapeutic cargo delivery requires standardizing exosome isolation methods along with their molecular and morphological characterization. With this aim, we isolated and characterized the exosomes released by human PRP platelets. Western blot analysis revealed that CaCl2-activated platelets (PLT-Exos-Ca2+) released more exosomes than non-activated ones (PLT-Exos). Moreover, PLT-Exos-Ca2+ exhibited a molecular signature that meets the most up-to-date biochemical criteria for platelet-derived exosomes and possessed morphological features typical of exosomes as assessed by transmission electron microscopy. Array analysis of 105 analytes including growth factors and cytokines showed that PLT-Exos-Ca2+ exhibited lower levels of most analytes compared to PLT-Exos, but relatively higher levels of those consistently validated as components of the protein cargo of platelet exosomes. In summary, the present study provides new insights into the molecular composition of human platelet-derived exosomes and validates a method for isolating highly pure platelet exosomes as a basis for future preclinical studies in regenerative medicine and drug delivery.
Keywords: cytokines; exosome markers; growth factors; human platelets; platelet-derived-exosomes; platelet-rich plasma.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Delgado D., Bilbao A.M., Beitia M., Garate A., Sánchez P., González-Burguera I., Isasti A., De Jesús M.L., Zuazo-Ibarra J., Montilla A., et al. Effects of platelet-rich plasma on cellular populations of the central nervous system: The influence of donor age. Int. J. Mol. Sci. 2021;22:1725. doi: 10.3390/ijms22041725. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

