Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing
- PMID: 35270041
- PMCID: PMC8911204
- DOI: 10.3390/ijms23052899
Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing
Abstract
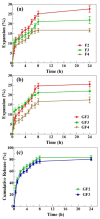
Biopolymer-based antibacterial films are attractive materials for wound dressing application because they possess chemical, mechanical, exudate absorption, drug delivery, antibacterial, and biocompatible properties required to support wound healing. Herein, we fabricated and characterized films composed of arabinoxylan (AX) and sodium alginate (SA) loaded with gentamicin sulfate (GS) for application as a wound dressing. The FTIR, XRD, and thermal analyses show that AX, SA, and GS interacted through hydrogen bonding and were thermally stable. The AXSA film displays desirable wound dressing characteristics: transparency, uniform thickness, smooth surface morphology, tensile strength similar to human skin, mild water/exudate uptake capacity, water transmission rate suitable for wound dressing, and excellent cytocompatibility. In Franz diffusion release studies, >80% GS was released from AXSA films in two phases in 24 h following the Fickian diffusion mechanism. In disk diffusion assay, the AXSA films demonstrated excellent antibacterial effect against E.coli, S. aureus, and P. aeruginosa. Overall, the findings suggest that GS-loaded AXSA films hold potential for further development as antibacterial wound dressing material.
Keywords: antibacterial dressing; arabinoxylan; drug delivery; sodium alginate; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Saghazadeh S., Rinoldi C., Schot M., Kashaf S.S., Sharifi F., Jalilian E., Nuutila K., Giatsidis G., Mostafalu P., Derakhshandeh H., et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018;127:138–166. doi: 10.1016/j.addr.2018.04.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

