In Vivo Imaging and Quantification of Carbon Tracer Dynamics in Nodulated Root Systems of Pea Plants
- PMID: 35270102
- PMCID: PMC8912644
- DOI: 10.3390/plants11050632
In Vivo Imaging and Quantification of Carbon Tracer Dynamics in Nodulated Root Systems of Pea Plants
Abstract
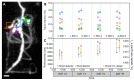
Legumes associate with root colonizing rhizobia that provide fixed nitrogen to its plant host in exchange for recently fixed carbon. There is a lack of understanding of how individual plants modulate carbon allocation to a nodulated root system as a dynamic response to abiotic stimuli. One reason is that most approaches are based on destructive sampling, making quantification of localised carbon allocation dynamics in the root system difficult. We established an experimental workflow for routinely using non-invasive Positron Emission Tomography (PET) to follow the allocation of leaf-supplied 11C tracer towards individual nodules in a three-dimensional (3D) root system of pea (Pisum sativum). Nitrate was used for triggering a reduction of biological nitrogen fixation (BNF), which was expected to rapidly affect carbon allocation dynamics in the root-nodule system. The nitrate treatment led to a decrease in 11C tracer allocation to nodules by 40% to 47% in 5 treated plants while the variation in control plants was less than 11%. The established experimental pipeline enabled for the first time that several plants could consistently be labelled and measured using 11C tracers in a PET approach to quantify C-allocation to individual nodules following a BNF reduction. Our study demonstrates the strength of using 11C tracers in a PET approach for non-invasive quantification of dynamic carbon allocation in several growing plants over several days. A major advantage of the approach is the possibility to investigate carbon dynamics in small regions of interest in a 3D system such as nodules in comparison to whole plant development.
Keywords: 11C; PET; biological nitrogen fixation; legumes; nitrate inhibition; radiotracer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Rubiales D., Mikic A. Introduction: Legumes in Sustainable Agriculture. Crit. Rev. Plant Sci. 2015;34:2–3. doi: 10.1080/07352689.2014.897896. - DOI
-
- Stagnari F., Maggio A., Galieni A., Pisante M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017;4:13. doi: 10.1186/s40538-016-0085-1. - DOI
-
- Kouchi H., Akao S., Yoneyama T. Respiratory utilization of C-13-labeled photosynthate in nodulated root systems of soybean plants. J. Exp. Bot. 1986;37:985–993. doi: 10.1093/jxb/37.7.985. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

