Live cell tracking of macrophage efferocytosis during Drosophila embryo development in vivo
- PMID: 35271315
- PMCID: PMC7612538
- DOI: 10.1126/science.abl4430
Live cell tracking of macrophage efferocytosis during Drosophila embryo development in vivo
Abstract
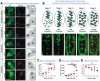
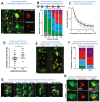
Apoptosis of cells and their subsequent removal through efferocytosis occurs in nearly all tissues during development, homeostasis, and disease. However, it has been difficult to track cell death and subsequent corpse removal in vivo. We developed a genetically encoded fluorescent reporter, CharON (Caspase and pH Activated Reporter, Fluorescence ON), that could track emerging apoptotic cells and their efferocytic clearance by phagocytes. Using Drosophila expressing CharON, we uncovered multiple qualitative and quantitative features of coordinated clearance of apoptotic corpses during embryonic development. When confronted with high rates of emerging apoptotic corpses, the macrophages displayed heterogeneity in engulfment behaviors, leading to some efferocytic macrophages carrying high corpse burden. Overburdened macrophages were compromised in clearing wound debris. These findings reveal known and unexpected features of apoptosis and macrophage efferocytosis in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures


Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

