The C terminus of the mycobacterium ESX-1 secretion system substrate ESAT-6 is required for phagosomal membrane damage and virulence
- PMID: 35271388
- PMCID: PMC8931374
- DOI: 10.1073/pnas.2122161119
The C terminus of the mycobacterium ESX-1 secretion system substrate ESAT-6 is required for phagosomal membrane damage and virulence
Abstract
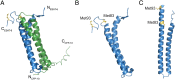
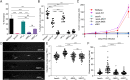
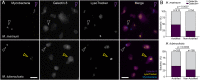
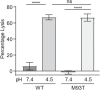
SignificanceTuberculosis (TB), an ancient disease of humanity, continues to be a major cause of worldwide death. The causative agent of TB, Mycobacterium tuberculosis, and its close pathogenic relative Mycobacterium marinum, initially infect, evade, and exploit macrophages, a major host defense against invading pathogens. Within macrophages, mycobacteria reside within host membrane-bound compartments called phagosomes. Mycobacterium-induced damage of the phagosomal membranes is integral to pathogenesis, and this activity has been attributed to the specialized mycobacterial secretion system ESX-1, and particularly to ESAT-6, its major secreted protein. Here, we show that the integrity of the unstructured ESAT-6 C terminus is required for macrophage phagosomal damage, granuloma formation, and virulence.
Keywords: ESAT-6; ESX-1; phagosomal damage; virulence.
Conflict of interest statement
Competing interest statement: R.B. is a coauthor on a 2018 paper with Eric Rubin, one of the reviewers of this paper.
Figures


References
-
- Pym A. S., Brodin P., Brosch R., Huerre M., Cole S. T., Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46, 709–717 (2002). - PubMed
-
- Brodin P., Rosenkrands I., Andersen P., Cole S. T., Brosch R., ESAT-6 proteins: Protective antigens and virulence factors? Trends Microbiol. 12, 500–508 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

