A coherent FOXO3-SNAI2 feed-forward loop in autophagy
- PMID: 35271390
- PMCID: PMC8931368
- DOI: 10.1073/pnas.2118285119
A coherent FOXO3-SNAI2 feed-forward loop in autophagy
Abstract
SignificanceUnderstanding autophagy regulation is instrumental in developing therapeutic interventions for autophagy-associated disease. Here, we identified SNAI2 as a regulator of autophagy from a genome-wide screen in HeLa cells. Upon energy stress, SNAI2 is transcriptionally activated by FOXO3 and interacts with FOXO3 to form a feed-forward regulatory loop to reinforce the expression of autophagy genes. Of note, SNAI2-increased FOXO3-DNA binding abrogates CRM1-dependent FOXO3 nuclear export, illuminating a pivotal role of DNA in the nuclear retention of nucleocytoplasmic shuttling proteins. Moreover, a dFoxO-Snail feed-forward loop regulates both autophagy and cell size in Drosophila, suggesting this evolutionarily conserved regulatory loop is engaged in more physiological activities.
Keywords: Drosophila; FOXO3; SNAI2; dFoxO; snail.
Conflict of interest statement
The authors declare no competing interest.
Figures


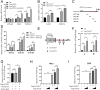


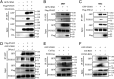

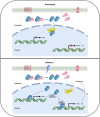
Similar articles
-
A conserved interplay between FOXO and SNAI/snail in autophagy.Autophagy. 2022 Nov;18(11):2759-2760. doi: 10.1080/15548627.2022.2063559. Epub 2022 Apr 15. Autophagy. 2022. PMID: 35422194 Free PMC article.
-
Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction.J Biol Chem. 2017 Aug 18;292(33):13774-13783. doi: 10.1074/jbc.M117.791483. Epub 2017 Jul 13. J Biol Chem. 2017. PMID: 28705935 Free PMC article.
-
Glucocorticoid-induced leucine zipper (GILZ) promotes the nuclear exclusion of FOXO3 in a Crm1-dependent manner.J Biol Chem. 2010 Feb 19;285(8):5594-605. doi: 10.1074/jbc.M109.068346. Epub 2009 Dec 14. J Biol Chem. 2010. PMID: 20018851 Free PMC article.
-
Understanding Forkhead box class O function: lessons from Drosophila melanogaster.Antioxid Redox Signal. 2011 Feb 15;14(4):635-47. doi: 10.1089/ars.2010.3407. Epub 2010 Oct 20. Antioxid Redox Signal. 2011. PMID: 20618068 Review.
-
FOXO3: at the crossroads of metabolic, inflammatory, and tumorigenic remodeling in the colon.Am J Physiol Gastrointest Liver Physiol. 2024 Mar 1;326(3):G247-G251. doi: 10.1152/ajpgi.00201.2023. Epub 2024 Jan 9. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38193202 Free PMC article. Review.
Cited by
-
Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin.Int J Mol Sci. 2023 Sep 5;24(18):13703. doi: 10.3390/ijms241813703. Int J Mol Sci. 2023. PMID: 37762015 Free PMC article.
-
Toxoplasma gondii inhibits the expression of autophagy-related genes through AKT-dependent inactivation of the transcription factor FOXO3a.mBio. 2023 Aug 31;14(4):e0079523. doi: 10.1128/mbio.00795-23. Epub 2023 Jun 30. mBio. 2023. PMID: 37387601 Free PMC article.
-
Contribution of Autophagy to Epithelial Mesenchymal Transition Induction during Cancer Progression.Cancers (Basel). 2024 Feb 16;16(4):807. doi: 10.3390/cancers16040807. Cancers (Basel). 2024. PMID: 38398197 Free PMC article. Review.
-
A conserved interplay between FOXO and SNAI/snail in autophagy.Autophagy. 2022 Nov;18(11):2759-2760. doi: 10.1080/15548627.2022.2063559. Epub 2022 Apr 15. Autophagy. 2022. PMID: 35422194 Free PMC article.
-
Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors.Antioxidants (Basel). 2024 Aug 7;13(8):958. doi: 10.3390/antiox13080958. Antioxidants (Basel). 2024. PMID: 39199205 Free PMC article.
References
-
- Mizushima N., Autophagy: Process and function. Genes Dev. 21, 2861–2873 (2007). - PubMed
-
- Klionsky D. J., Autophagy: From phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 (2007). - PubMed
-
- López-Otín C., Kroemer G., Hallmarks of health. Cell 184, 33–63 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

