Cross-linkers at growing microtubule ends generate forces that drive actin transport
- PMID: 35271394
- PMCID: PMC8931237
- DOI: 10.1073/pnas.2112799119
Cross-linkers at growing microtubule ends generate forces that drive actin transport
Abstract
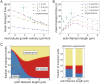
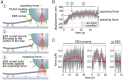
SignificanceComplex cellular processes such as cell migration require coordinated remodeling of both the actin and the microtubule cytoskeleton. The two networks for instance exert forces on each other via active motor proteins. Here we show that, surprisingly, coupling via passive cross-linkers can also result in force generation. We specifically study the transport of actin filaments by growing microtubule ends. We show by cell-free reconstitution experiments, computer simulations, and theoretical modeling that this transport is driven by the affinity of the cross-linker for the chemically distinct microtubule tip region. Our work predicts that growing microtubules could potentially rapidly relocate newly nucleated actin filaments to the leading edge of the cell and thus boost migration.
Keywords: cell biophysics; crosstalk; cytoskeleton; kinetic Monte Carlo simulations; self-organization.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Dogterom M., Kerssemakers J. W., Romet-Lemonne G., Janson M. E., Force generation by dynamic microtubules. Curr. Opin. Cell Biol. 17, 67–74 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

