The Eph receptor A4 plays a role in demyelination and depression-related behavior
- PMID: 35271507
- PMCID: PMC9012277
- DOI: 10.1172/JCI152187
The Eph receptor A4 plays a role in demyelination and depression-related behavior
Erratum in
-
The Eph receptor A4 plays a role in demyelination and depression-related behavior.J Clin Invest. 2022 May 16;132(10):e161559. doi: 10.1172/JCI161559. J Clin Invest. 2022. PMID: 35575094 Free PMC article. No abstract available.
Abstract
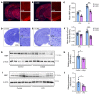
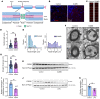
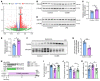
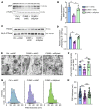
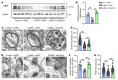
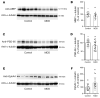
Proper myelination of axons is crucial for normal sensory, motor, and cognitive function. Abnormal myelination is seen in brain disorders such as major depressive disorder (MDD), but the molecular mechanisms connecting demyelination with the pathobiology remain largely unknown. We observed demyelination and synaptic deficits in mice exposed to either chronic, unpredictable mild stress (CUMS) or LPS, 2 paradigms for inducing depression-like states. Pharmacological restoration of myelination normalized both synaptic deficits and depression-related behaviors. Furthermore, we found increased ephrin A4 receptor (EphA4) expression in the excitatory neurons of mice subjected to CUMS, and shRNA knockdown of EphA4 prevented demyelination and depression-like behaviors. These animal data are consistent with the decrease in myelin basic protein and the increase in EphA4 levels we observed in postmortem brain samples from patients with MDD. Our results provide insights into the etiology of depressive symptoms in some patients and suggest that inhibition of EphA4 or the promotion of myelination could be a promising strategy for treating depression.
Keywords: Cell Biology; Demyelinating disorders; Molecular biology; Neuroscience.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

