Digital Therapeutic Device for Urinary Incontinence: A Randomized Controlled Trial
- PMID: 35271539
- PMCID: PMC8936159
- DOI: 10.1097/AOG.0000000000004725
Digital Therapeutic Device for Urinary Incontinence: A Randomized Controlled Trial
Abstract
Objective: To evaluate whether pelvic floor muscle training using a motion-based digital intravaginal device is more effective than home pelvic floor muscle training for treatment of stress or stress-predominant mixed urinary incontinence (UI).
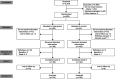
Methods: In a remote, virtually executed 8-week prospective randomized controlled superiority trial, women with stress or stress-predominant mixed UI were randomized to pelvic floor muscle training using a motion-based digital therapeutic device or a home training program using written and narrated instructions. Primary outcomes were change in UDI-6 (Urogenital Distress Inventory, Short Form) score and stress urinary incontinence (SUI) episodes on a 3-day bladder diary. A sample size of 139 per group (n=278) was planned to meet the power analysis requirements for the UDI-6 score (n=278) and the bladder diary (n=78). Prespecified secondary outcomes included quality-of-life surveys and adherence reporting.
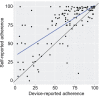
Results: From September 2020 to March 2021, 5,353 participants were screened, and 363 were randomized: 182 in the intervention and 181 in the control group. There were no baseline clinicodemographic differences between groups. The mean change in UDI-6 score was significantly greater for the intervention group compared with the control group (18.8 vs 14.7, P=.01). The median (interquartile range) number of SUI episodes on the 3-day bladder diary was significantly reduced from 5 (3-8) and 5 (3-8) episodes to 1 (0-3) and 2 (1-4) (P=.005) in the intervention group compared with control group, respectively. A significantly greater number of participants in the intervention group than in the control group reported they were "much improved" or "very much improved" on the PGI-I (Patient Global Impression of Improvement) (63/143 [44.1% vs 45/156 [28.8%], odds ratio 1.94, 95% CI 1.21-3.15). There were no device-related severe adverse events.
Conclusion: In this all-remote, virtually conducted trial, pelvic floor muscle training guided by a motion-based digital therapeutic device resulted in significantly improved UI symptoms and reduction of UI episodes compared with a home training program.
Clinical trial registration: ClinicalTrials.gov, NCT04508153.
Funding source: Renovia Inc.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Financial Disclosure Milena M. Weinstein disclosed receiving funding from UpToDate. Gena Dunivan disclosed that money was paid to her institution from Viveve and Renovia. Travel reimbursement was received from ABOG and ACOG. Holly E. Richter's institution received funding from the NIDDK, NICHD, PCORI, Allergan, Pelvalon, and Renovia. She received royalties from UpToDate and travel reimbursement related to work on the editorial boards of IUJ and ACOG. Dr. Richter serves on the Board of Directors of the American Urogynecologic Society and the World-Wide Fistula Fund. She is on the Data Safety Monitoring Board for BlueWind. The other authors did not report any potential conflicts of interest.
Figures

Comment in
-
In women with stress UI, device-guided pelvic floor muscle training safely improved symptoms more than self-guided training.Ann Intern Med. 2022 Jul;175(7):JC79. doi: 10.7326/J22-0044. Epub 2022 Jul 5. Ann Intern Med. 2022. PMID: 35785541
-
Digital Therapeutic Device for Urinary Incontinence: A Randomized Controlled Trial.Obstet Gynecol. 2022 Jul 1;140(1):136. doi: 10.1097/AOG.0000000000004843. Obstet Gynecol. 2022. PMID: 35849471 No abstract available.

