Predictive Value of Isolated Symptoms for Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children Tested During Peak Circulation of the Delta Variant
- PMID: 35271694
- PMCID: PMC8992302
- DOI: 10.1093/cid/ciac112
Predictive Value of Isolated Symptoms for Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children Tested During Peak Circulation of the Delta Variant
Abstract
Background: Coronavirus disease 2019 (COVID-19) testing policies for symptomatic children attending US schools or daycare vary, and whether isolated symptoms should prompt testing is unclear. We evaluated children presenting for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing to determine if the likelihood of having a positive SARS-CoV-2 test differed between participants with 1 symptom vs ≥2 symptoms, and to examine the predictive capability of isolated symptoms.
Methods: Participants aged <18 years presenting for clinical SARS-CoV-2 molecular testing in 6 sites in urban/suburban/rural Georgia (July-October, 2021; Delta variant predominant) were queried about individual symptoms. Participants were classified into 3 groups: asymptomatic, 1 symptom only, or ≥2 symptoms. SARS-CoV-2 test results and clinical characteristics of the 3 groups were compared. Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for isolated symptoms were calculated by fitting a saturated Poisson model.
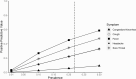
Results: Of 602 participants, 21.8% tested positive and 48.7% had a known or suspected close contact. Children reporting 1 symptom (n = 82; odds ratio [OR], 6.00 [95% confidence interval {CI}, 2.70-13.33]) and children reporting ≥2 symptoms (n = 365; OR, 5.25 [95% CI, 2.66-10.38]) were significantly more likely to have a positive COVID-19 test than asymptomatic children (n = 155), but they were not significantly different from each other (OR, 0.88 [95% CI, .52-1.49]). Sensitivity and PPV were highest for isolated fever (33% and 57%, respectively), cough (25% and 32%), and sore throat (21% and 45%); headache had low sensitivity (8%) but higher PPV (33%). Sensitivity and PPV of isolated congestion/rhinorrhea were 8% and 9%, respectively.
Conclusions: With high Delta variant prevalence, children with isolated symptoms were as likely as those with multiple symptoms to test positive for COVID-19. Isolated fever, cough, sore throat, or headache, but not congestion/rhinorrhea, offered the highest predictive value.
Keywords: COVID-19; SARS-CoV-2; children; symptom; testing.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. C. A. R.’s institution has received funds to conduct clinical research unrelated to this manuscript from BioFire, GSK, MedImmune, Micron, Janssen, Merck, Moderna, Novavax, PaxVax, Pfizer, Regeneron, and Sanofi-Pasteur. She is coinventor of patented respiratory syncytial virus vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, and has received royalties; and is named in the following patents: “Chimeric RSV, immunogenic compositions, and methods of use,” International PCT application number PCT/US2016/058976, filed 28 December 2016 by Emory University, and “Osteopontin as a biomarker of COVID-19 and MIS-C disease,” US patent application number 63/229,718, filed 5 August 2021 by Emory University. J. M. L. has received speaker honorarium from the American College of Allergy, Asthma, and Immunology and has served on advisory boards for GlaxoSmithKline and Regeneron. G. S. M. reports payments to Emory University from the National Institute of Biomedical Imaging and Bioengineering, NIH (U54-027690), outside the submitted work. N. R. P. has served as a subject matter expert for the Massachusetts Department of Public Health on evaluation and implementation of testing for SARS-CoV-2 infection. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Massachusetts Department of Elementary and Secondary Eduction. COVID-19 testing program, COVID-19 protocols flowcharts. 2021. Available at: https://www.doe.mass.edu/covid19/on-desktop.html.
-
- Georgia Department of Public Health. Changes and updates: COVID-19 guidance for Georgia K-12 schools and school-based programs. 2020. Available at: https://www.georgiainsights.com/uploads/1/2/2/2/122221993/school_updates.... Accessed 15 November 2021.
-
- San Mateo County Health, Public Health, Policy, and Planning. COVID-19 recommendations checklist for K-12 schools and other school-based programs. 2021. Available at: https://www.smchealth.org/sites/main/files/file-attachments/covid-19_rec.... Accessed 15 November 2021.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

