Humoral Responses Against Variants of Concern by COVID-19 mRNA Vaccines in Immunocompromised Patients
- PMID: 35271706
- PMCID: PMC8914885
- DOI: 10.1001/jamaoncol.2022.0446
Humoral Responses Against Variants of Concern by COVID-19 mRNA Vaccines in Immunocompromised Patients
Abstract
Importance: There are limited comparative data on the durability of neutralizing antibody (nAb) responses elicited by messenger RNA (mRNA) vaccines against the SARS-CoV-2 variants of concern (VOCs) in immunocompromised patients and healthy controls.
Objective: To assess the humoral responses after vaccination with BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccines.
Design, setting, and participants: In this prospective, longitudinal monocentric comparative effectiveness study conducted at the Lausanne University Hospital, binding IgG anti-spike antibody and nAb levels were measured at 1 week, 1 month, 3 months, and 6 months after vaccination with mRNA-1273 (24.6% of participants) or BNT162b2 (75.3% of participants).
Interventions: All participants received 2 doses of either mRNA-1273 or BNT162b2 vaccines 4 to 6 weeks apart.
Main outcomes and measures: The primary outcome of the study was the persistence of nAb responses against the original, nonvariant SARS-CoV-2 (2019-nCoV) and different VOCs at 6 months after vaccination. Key secondary outcomes were associations of the type of mRNA vaccine, the underlying disease, and the treatment with the response to vaccination.
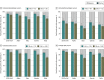
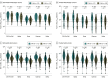
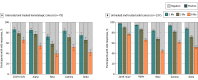
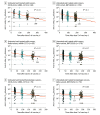
Results: Among the 841 participants enrolled between January 14 and August 8, 2021, the patient population comprised 637 participants (mean [SD] age, 61.8 [13.7] years; 386 [60.6%] female), and the healthy control population comprised 204 participants (mean [SD] age, 45.9 [12.0] years; 144 [70.6%] female). There were 399 patients with solid cancers, 101 with hematologic cancers, 38 with solid organ transplants, 99 with autoimmune diseases, and 204 healthy controls. More than 15 000 nAb determinations were performed against the original, nonvariant 2019-nCoV and the Alpha, Beta, Gamma, and Delta variants. The proportions of nAbs and their titers decreased in all study groups at 6 months after vaccination, with the greatest decreases for the Beta and Delta variants. For Beta, the proportion decreased to a median (SE) of 39.2% (5.5%) in those with hematologic cancers, 44.8% (2.7%) in those with solid cancers, 23.1% (8.3%) in those with solid organ transplants, and 22.7% (4.8%) in those with autoimmune diseases compared with 52.1% (4.2%) in healthy controls. For Delta, the proportions decreased to 41.8% (5.6%) in participants with hematologic cancer, 51.9% (2.7%) in those with solid cancers, 26.9% (8.7%) in those with solid organ transplants, and 30.7% (5.3%) in those with autoimmune diseases compared with 56.9% (4.1%) healthy controls. Neutralizing antibody titers decreased 3.5- to 5-fold between month 1 and month 6, and the estimated duration of response was greater and more durable among those participants vaccinated with mRNA-1273. In participants with solid cancers, the estimated duration of nAbs against the Beta variant was 221 days with mRNA-1273 and 146 days with BNT162b2, and against the Delta variant, it was 226 days with mRNA-1273 and 161 with BNT162b2. The estimated duration of nAbs in participants with hematologic cancers was 113 and 127 days against Beta and Delta variants, respectively.
Conclusions and relevance: This comparative effectiveness study suggests that approximately half of patients with hematologic cancers and solid cancers, about 70% of patients with solid organ transplants or autoimmune diseases, and 40% of healthy controls have lost nAbs against the circulating VOCs at 6 months after vaccination. These findings may be helpful for developing the best boosting vaccination schedule especially in immunocompromised patients.
Conflict of interest statement
Figures

References
-
- Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020;annrheumdis-2020-218946. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

