Risk of variceal hemorrhage and pretransplant mortality in children with biliary atresia
- PMID: 35271743
- PMCID: PMC9378352
- DOI: 10.1002/hep.32451
Risk of variceal hemorrhage and pretransplant mortality in children with biliary atresia
Abstract
Background and aims: The natural history of gastroesophageal variceal hemorrhage (VH) in biliary atresia (BA) is not well characterized. We analyzed risk factors, incidence, and outcomes of VH in a longitudinal multicenter study.
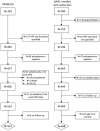
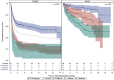
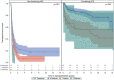
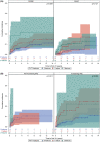
Approach and results: Participants enrolled in either an incident (Prospective Database of Infants with Cholestasis [PROBE]) or prevalent (Biliary Atresia Study of Infants and Children [BASIC]) cohort of BA were included. Variceal hemorrhage (VH) was defined based on gastrointestinal bleeding in the presence of varices accompanied by endoscopic or nontransplant surgical intervention. Cumulative incidence of VH and transplant-free survival was compared based on features of portal hypertension (e.g., splenomegaly, thrombocytopenia) and clinical parameters at baseline in each cohort (PROBE: 1.5 to 4.5 months after hepatoportoenterostomy [HPE]; BASIC: at enrollment > 3 years of age). Analyses were conducted on 869 children with BA enrolled between June 2004 and December 2020 (521 in PROBE [262 (51%) with a functioning HPE] and 348 in BASIC). The overall incidence of first observed VH at 5 years was 9.4% (95% CI: 7.0-12.4) in PROBE and 8.0% (5.2-11.5) in BASIC. Features of portal hypertension, platelet count, total bilirubin, aspartate aminotransferase (AST), albumin, and AST-to-platelet ratio index at baseline were associated with an increased risk of subsequent VH in both cohorts. Transplant-free survival at 5 years was 45.1% (40.5-49.6) in PROBE and 79.2% (74.1-83.4) in BASIC. Two (2.5%) of 80 participants who had VH died, whereas 10 (12.5%) underwent transplant within 6 weeks of VH.
Conclusions: The low risk of VH and associated mortality in children with BA needs to be considered in decisions related to screening for varices and primary prophylaxis of VH.
© 2022 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Miethke consults for Mirum and Metacrine. Dr. Ng consults for Albireo. Dr. Murray consults for Gilead and Albireo. Dr. Loomes consults for and received grants from Mirum and Albireo. Dr. Molleston received grants from Mirum, AbbVie, Gilead, and Albireo. Dr. Rosenthal consults for and received grants from Takeda/Vertex, Gilead, AbbVie, Retrophin, Albireo, Mirum, and Travere. He consults for Audentes and Dicerna. He received grants from Merck and Arrowhead. Dr. Karpen consults for Albireo, Intercept, and Mirum. Dr. Sokol consults for Albireo and Mirum.
Figures

References
-
- Duche M, Ducot B, Ackermann O, Guerin F, Jacquemin E, Bernard O. Portal hypertension in children: high‐risk varices, primary prophylaxis and consequences of bleeding. J Hepatol. 2017;66:320 27. - PubMed
-
- Miga D, Sokol RJ, Mackenzie T, Narkewicz MR, Smith D, Karrer FM. Survival after first esophageal variceal hemorrhage in patients with biliary atresia. J Pediatr. 2001;139:291–6. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK062481/DK/NIDDK NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- UL1 TR001857/TR/NCATS NIH HHS/United States
- U01 DK062436/DK/NIDDK NIH HHS/United States
- U01 DK062466/DK/NIDDK NIH HHS/United States
- U01 DK084575/DK/NIDDK NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- U01 DK062456/DK/NIDDK NIH HHS/United States
- U01 DK103149/DK/NIDDK NIH HHS/United States
- U24 DK062456/DK/NIDDK NIH HHS/United States
- UL1 TR000130/TR/NCATS NIH HHS/United States
- U01 DK103140/DK/NIDDK NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- U01 DK084538/DK/NIDDK NIH HHS/United States
- U01 DK062453/DK/NIDDK NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- U01 DK103135/DK/NIDDK NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- U01 DK084536/DK/NIDDK NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- U01 DK062445/DK/NIDDK NIH HHS/United States
- K08 HS026510/HS/AHRQ HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- U01 DK062500/DK/NIDDK NIH HHS/United States
- U01 DK062497/DK/NIDDK NIH HHS/United States

