Kidney function in tenofovir disoproxil fumarate-based oral pre-exposure prophylaxis users: a systematic review and meta-analysis of published literature and a multi-country meta-analysis of individual participant data
- PMID: 35271825
- PMCID: PMC8964504
- DOI: 10.1016/S2352-3018(22)00004-2
Kidney function in tenofovir disoproxil fumarate-based oral pre-exposure prophylaxis users: a systematic review and meta-analysis of published literature and a multi-country meta-analysis of individual participant data
Abstract
Background: Previous WHO guidance on tenofovir disoproxil fumarate-based oral pre-exposure prophylaxis (PrEP) suggests measuring creatinine levels at PrEP initiation and regularly afterwards, which might represent barriers to PrEP implementation and uptake. We aimed to systematically review published literature on kidney toxicity among tenofovir disoproxil fumarate-based oral PrEP users and conducted an individual participant data meta-analysis (IPDMA) on kidney function among PrEP users in a global implementation project dataset.
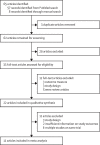
Methods: In this systematic review and meta-analysis we searched PubMed up to June 30, 2021, for randomised controlled trials (RCTs) or cohort studies that reported on graded kidney-related adverse events among oral PrEP users (tenofovir disoproxil fumarate-based PrEP alone or in combination with emtricitabine or lamivudine). We extracted summary data and conducted meta-analyses with random-effects models to estimate relative risks of grade 1 and higher and grade 2 and higher kidney-related adverse events, measured by elevated serum creatinine or decline in estimated creatinine clearance or estimated glomerular filtration rate. The IPDMA included (largely unpublished) individual participant data from 17 PrEP implementation projects and two RCTs. Estimated baseline creatinine clearance and creatinine clearance change after initiation were described by age, gender, and comorbidities. We used random-effects regressions to estimate the risk in decline of creatinine clearance to less than 60 mL/min.
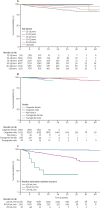
Findings: We identified 62 unique records and included 17 articles reporting on 11 RCTs with 13 523 participants in meta-analyses. PrEP use was associated with increased risk of grade 1 and higher kidney adverse events (pooled odds ratio [OR] 1·49, 95% CI 1·22-1·81; I2=25%) and grade 2 and higher events (OR 1·75, 0·68-4·49; I2=0%), although the grade 2 and higher association was not statistically significant and events were rare (13 out of 6764 in the intervention group vs six out of 6782 in the control group). The IPDMA included 18 676 individuals from 15 countries (1453 [7·8%] from RCTs) and 79 (0·42%) had a baseline estimated creatinine clearance of less than 60 mL/min (increasing proportions with increasing age). Longitudinal analyses included 14 368 PrEP users and 349 (2·43%) individuals had a decline to less than 60 mL/min creatinine clearance, with higher risks associated with increasing age and baseline creatinine clearance of 60·00-89·99 mL/min (adjusted hazard ratio [aHR] 8·49, 95% CI 6·44-11·20) and less than 60 mL/min (aHR 20·83, 12·83-33·82).
Interpretation: RCTs suggest that risks of kidney-related adverse events among tenofovir disoproxil fumarate-based oral PrEP users are increased but generally mild and small. Our global PrEP user analysis found varying risks by age and baseline creatinine clearance. Kidney function screening and monitoring might focus on older individuals, those with baseline creatinine clearance of less than 90 mL/min, and those with kidney-related comorbidities. Less frequent or optional screening among younger individuals without kidney-related comorbidities may reduce barriers to PrEP implementation and use.
Funding: Unitaid, Bill & Melinda Gates Foundation, WHO.
© 2022 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products, or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests J-MM declares that his institution has received grants from Gilead Sciences; receiving consulting fees from Gilead Sciences, Merck & Co, and ViiV Healthcare; and payments for expert testimony from Merck & Co, unrelated to the present work. KG declares receipt of travel grants by IAS and WHO to attend meetings unrelated to the present work. All other authors declare no competing interests.
Figures

Comment in
-
Real-world safety of pre-exposure prophylaxis for HIV.Lancet HIV. 2022 Apr;9(4):e225-e226. doi: 10.1016/S2352-3018(22)00058-3. Epub 2022 Mar 7. Lancet HIV. 2022. PMID: 35271827 No abstract available.
References
-
- Chou R, Evans C, Hoverman A, et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:2214–2230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

