COVID-19 and neurological sequelae: Vitamin D as a possible neuroprotective and/or neuroreparative agent
- PMID: 35271880
- PMCID: PMC8898786
- DOI: 10.1016/j.lfs.2022.120464
COVID-19 and neurological sequelae: Vitamin D as a possible neuroprotective and/or neuroreparative agent
Abstract
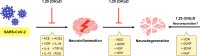
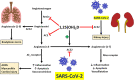
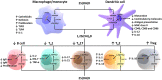
SARS-CoV-2, the etiological agent of the current COVID-19 pandemic, belongs to a broad family of coronaviruses that also affect humans. SARS-CoV-2 infection usually leads to bilateral atypical pneumonia with significant impairment of respiratory function. However, the infectious capacity of SARS-CoV-2 is not limited to the respiratory system, but may also affect other vital organs such as the brain. The central nervous system is vulnerable to cell damage via direct invasion or indirect virus-related effects leading to a neuroinflammatory response, processes possibly associated with a decrease in the activity of angiotensin II converting enzyme (ACE2), the canonical cell-surface receptor for SARS-CoV-2. This enzyme regulates neuroprotective and neuroimmunomodulatory functions and can neutralize both inflammation and oxidative stress generated at the cellular level. Furthermore, there is evidence of an association between vitamin D deficiency and predisposition to the development of severe forms of COVID-19, with its possible neurological and neuropsychiatric sequelae: vitamin D has the ability to down-modulate the effects of neuroinflammatory cytokines, among other anti-inflammatory/immunomodulatory effects, thus attenuating harmful consequences of COVID-19. This review critically analyzes current evidence supporting the notion that vitamin D may act as a neuroprotective and neuroreparative agent against the neurological sequelae of COVID-19.
Keywords: COVID-19; Neurodegeneration; Neuroinflammation; Neuroreparation; Sequelae; Vitamin D.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author(s) declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Putative mechanism of neurological damage in COVID-19 infection.Acta Neurobiol Exp (Wars). 2021;81(1):69-79. doi: 10.21307/ane-2021-008. Acta Neurobiol Exp (Wars). 2021. PMID: 33949163 Review.
-
Impact of level of vitamin D in the body on the severity of COVID-19 - review of the literature.Przegl Epidemiol. 2020;74(4):583-595. doi: 10.32394/pe.74.50. Przegl Epidemiol. 2020. PMID: 33860776 Review.
-
Protective role of vitamin D status against COVID-19: a mini-review.Endocrine. 2023 Feb;79(2):235-242. doi: 10.1007/s12020-022-03203-8. Epub 2022 Oct 18. Endocrine. 2023. PMID: 36258153 Free PMC article. Review.
-
Neuroimmunological Effect of Vitamin D on Neuropsychiatric Long COVID Syndrome: A Review.Nutrients. 2023 Aug 30;15(17):3802. doi: 10.3390/nu15173802. Nutrients. 2023. PMID: 37686834 Free PMC article. Review.
-
Novel CYP11A1-Derived Vitamin D and Lumisterol Biometabolites for the Management of COVID-19.Nutrients. 2022 Nov 11;14(22):4779. doi: 10.3390/nu14224779. Nutrients. 2022. PMID: 36432468 Free PMC article. Review.
Cited by
-
Aged brain and neuroimmune responses to COVID-19: post-acute sequelae and modulatory effects of behavioral and nutritional interventions.Immun Ageing. 2023 Apr 12;20(1):17. doi: 10.1186/s12979-023-00341-z. Immun Ageing. 2023. PMID: 37046272 Free PMC article. Review.
-
Vitamin D and COVID-19: Clinical Evidence and Immunological Insights.Life (Basel). 2025 Apr 30;15(5):733. doi: 10.3390/life15050733. Life (Basel). 2025. PMID: 40430160 Free PMC article. Review.
-
Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases.Nutrients. 2023 Feb 2;15(3):767. doi: 10.3390/nu15030767. Nutrients. 2023. PMID: 36771473 Free PMC article. Review.
-
Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy.Inflammopharmacology. 2024 Feb;32(1):249-271. doi: 10.1007/s10787-023-01383-x. Epub 2023 Nov 13. Inflammopharmacology. 2024. PMID: 37957515 Free PMC article. Review.
-
Vitamin D: A Role Also in Long COVID-19?Nutrients. 2022 Apr 13;14(8):1625. doi: 10.3390/nu14081625. Nutrients. 2022. PMID: 35458189 Free PMC article. Review.
References
-
- Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., Liu S.S., Zhang N.N., Li X.F., Xiong R., Guo Y., Deng Y.Q., Huang W.J., Liu Q., Liu Q.M., Shen Y.L., Zhou Y., Yang X., Zhao T.Y., Fan C.F., Zhou Y.S., Qin C.F., Wang Y.C. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28(1):124–133.e4. doi: 10.1016/j.chom.2020.05.020. - DOI - PMC - PubMed
-
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Kazmi S.A.J., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20202135. - DOI - PMC - PubMed
-
- Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., Gautier-Vargas G., Keller N., Kremer S., Fafi-Kremer S., Moulin B., Benotmane I., Caillard S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021;28(1):248–258. doi: 10.1111/ene.14491. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

