The comprehensive analysis of clinical trials registration for IgA nephropathy therapy on ClinicalTrials.gov
- PMID: 35272573
- PMCID: PMC8920363
- DOI: 10.1080/0886022X.2022.2048017
The comprehensive analysis of clinical trials registration for IgA nephropathy therapy on ClinicalTrials.gov
Abstract
Objectives: IgA Nephropathy (IgAN) is common chronic kidney disease with a high incidence. This study aims to analyze comprehensively therapeutic clinical trials for IgAN registered on ClinicalTrials.gov.
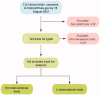
Methods: Therapeutic trials for IgAN registered on ClinicalTrials.gov. up to 15 August 2021 were obtained. The general characteristics, features of experimental design, treatment strategies, and some main inclusion criteria and outcome measures were accessed.
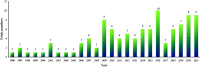
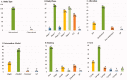
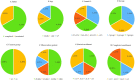
Results: A total of 104 therapeutic clinical trials for IgAN were extracted on ClinicalTrials.gov up to 15 August 2021. Most of these trials explored the treatment for primary IgAN confirmed by renal biopsy in adults. Only 9% of all selected trials had results. Forty-five percent of trials recruited 50 or fewer participants, and 73% were adults or older adults. 99% of trials were interventional studies, and of all the interventional trials, 70% of trials were randomized, and 68% exercised a parallel assignment of intervention model. Immunosuppression was the most studied for the treatment of IgAN. Moreover, many novel agents had been increasingly studied in recent years. Furthermore, the inclusion criteria and primary outcome measures in these trials were diverse, and the level of proteinuria and change of proteinuria levels were the most used as inclusion criteria and primary outcome, respectively.
Conclusions: The majority of therapeutic trials for IgAN were randomized, none masking and parallel-assignment interventional studies, primarily recruiting adult patients as research subjects. These trials had relatively small sample sizes and short observation. Thus, more large-scale, multicenter, and randomized controlled trials are still needed to improve the management for IgAN.
Keywords: ClinicalTrials.gov; IgA nephropathy; clinical trials; treatment.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Figures


References
-
- Cheung CK, Barratt J.. Should we STOP immunosuppression for IgA nephropathy? Long-term outcomes from the STOP-IgAN trial. Kidney Int. 2020;98(4):836–838. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
