Decreased Renal Gluconeogenesis Is a Hallmark of Chronic Kidney Disease
- PMID: 35273087
- PMCID: PMC8970457
- DOI: 10.1681/ASN.2021050680
Decreased Renal Gluconeogenesis Is a Hallmark of Chronic Kidney Disease
Abstract
Introduction: CKD is associated with alterations of tubular function. Renal gluconeogenesis is responsible for 40% of systemic gluconeogenesis during fasting, but how and why CKD affects this process and the repercussions of such regulation are unknown.
Methods: We used data on the renal gluconeogenic pathway from more than 200 renal biopsies performed on CKD patients and from 43 kidney allograft patients, and studied three mouse models, of proteinuric CKD (POD-ATTAC), of ischemic CKD, and of unilateral urinary tract obstruction. We analyzed a cohort of patients who benefitted from renal catheterization and a retrospective cohort of patients hospitalized in the intensive care unit.
Results: Renal biopsies of CKD and kidney allograft patients revealed a stage-dependent decrease in the renal gluconeogenic pathway. Two animal models of CKD and one model of kidney fibrosis confirm gluconeogenic downregulation in injured proximal tubule cells. This shift resulted in an alteration of renal glucose production and lactate clearance during an exogenous lactate load. The isolated perfused kidney technique in animal models and renal venous catheterization in CKD patients confirmed decreased renal glucose production and lactate clearance. In CKD patients hospitalized in the intensive care unit, systemic alterations of glucose and lactate levels were more prevalent and associated with increased mortality and a worse renal prognosis at follow-up. Decreased expression of the gluconeogenesis pathway and its regulators predicted faster histologic progression of kidney disease in kidney allograft biopsies.
Conclusion: Renal gluconeogenic function is impaired in CKD. Altered renal gluconeogenesis leads to systemic metabolic changes with a decrease in glucose and increase in lactate level, and is associated with a worse renal prognosis.
Keywords: chronic kidney disease; gluconeogenesis; metabolism.
Copyright © 2022 by the American Society of Nephrology.
Figures




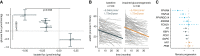
References
-
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR: Targeting the progression of chronic kidney disease. Nat Rev Nephrol 16: 269–288, 2020 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

