Signaling pathways and targeted therapy for myocardial infarction
- PMID: 35273164
- PMCID: PMC8913803
- DOI: 10.1038/s41392-022-00925-z
Signaling pathways and targeted therapy for myocardial infarction
Abstract
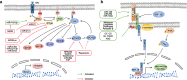
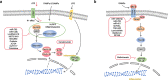
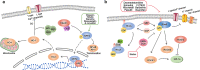
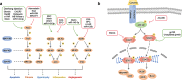
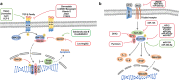
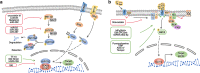
Although the treatment of myocardial infarction (MI) has improved considerably, it is still a worldwide disease with high morbidity and high mortality. Whilst there is still a long way to go for discovering ideal treatments, therapeutic strategies committed to cardioprotection and cardiac repair following cardiac ischemia are emerging. Evidence of pathological characteristics in MI illustrates cell signaling pathways that participate in the survival, proliferation, apoptosis, autophagy of cardiomyocytes, endothelial cells, fibroblasts, monocytes, and stem cells. These signaling pathways include the key players in inflammation response, e.g., NLRP3/caspase-1 and TLR4/MyD88/NF-κB; the crucial mediators in oxidative stress and apoptosis, for instance, Notch, Hippo/YAP, RhoA/ROCK, Nrf2/HO-1, and Sonic hedgehog; the controller of myocardial fibrosis such as TGF-β/SMADs and Wnt/β-catenin; and the main regulator of angiogenesis, PI3K/Akt, MAPK, JAK/STAT, Sonic hedgehog, etc. Since signaling pathways play an important role in administering the process of MI, aiming at targeting these aberrant signaling pathways and improving the pathological manifestations in MI is indispensable and promising. Hence, drug therapy, gene therapy, protein therapy, cell therapy, and exosome therapy have been emerging and are known as novel therapies. In this review, we summarize the therapeutic strategies for MI by regulating these associated pathways, which contribute to inhibiting cardiomyocytes death, attenuating inflammation, enhancing angiogenesis, etc. so as to repair and re-functionalize damaged hearts.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. J. Am. Coll. Cardiol. 2020;76:2980–2981. - PubMed
-
- Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat. Rev. Drug Discov. 2020;19:333–352. - PubMed
-
- Doenst T, et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;73:964–976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

