A multifaceted educational intervention improved anti-infectious measures but had no effect on mortality in patients with severe sepsis
- PMID: 35273276
- PMCID: PMC8913650
- DOI: 10.1038/s41598-022-07915-9
A multifaceted educational intervention improved anti-infectious measures but had no effect on mortality in patients with severe sepsis
Abstract
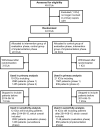
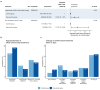
Sepsis is a major reason for preventable hospital deaths. A cluster-randomized controlled trial on an educational intervention did not show improvements of sepsis management or outcome. We now aimed to test an improved implementation strategy in a second intervention phase in which new intervention hospitals (former controls) received a multifaceted educational intervention, while controls (former intervention hospitals) only received feedback of quality indicators. Changes in outcomes from the first to the second intervention phase were compared between groups using hierarchical generalized linear models controlling for possible confounders. During the two phases, 19 control hospitals included 4050 patients with sepsis and 21 intervention hospitals included 2526 patients. 28-day mortality did not show significant changes between study phases in both groups. The proportion of patients receiving antimicrobial therapy within one hour increased in intervention hospitals, but not in control hospitals. Taking at least two sets of blood cultures increased significantly in both groups. During phase 2, intervention hospitals showed higher proportion of adequate initial antimicrobial therapy and de-escalation within 5 days. A survey among involved clinicians indicated lacking resources for quality improvement. Therefore, quality improvement programs should include all elements of sepsis guidelines and provide hospitals with sufficient resources for quality improvement.Trial registration: ClinicalTrials.gov, NCT01187134. Registered 23 August 2010, https://www.clinicaltrials.gov/ct2/show/study/NCT01187134 .
© 2022. The Author(s).
Conflict of interest statement
Dr. Schwarzkopf has nothing to disclose. Dr. Matthäus-Krämer has nothing to disclose. Dr. Thomas-Rüddel reports grants from BMBF, outside the submitted work. Dr. Rüddel has nothing to disclose. Dr. Poidinger has nothing to disclose. Dr. Bach has nothing to disclose. Dr. Gerlach has nothing to disclose. Dr. Gründling reports grants from BMBF, outside the submitted work. Dr. Lindner has nothing to disclose. Dr. Scheer has nothing to disclose. Dr. Simon reports personal fees from InfectoPharm, outside the submitted work. Dr. Weiss has nothing to disclose. Dr. Reinhart is shareholder with less of 0.5% of InflaRx NV a Jena /Germany based Biotech Company that evaluates a immunmodulatory approach for the adjunctive treatment of COVID-19. Dr. Bloos reports grants from German Federal Ministry of Education and Research, during the conduct of the study; personal fees from Baxter, outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

