A Physiologically Based Pharmacokinetic Model for Studying the Biowaiver Risk of Biopharmaceutics Classification System Class I Drugs With Rapid Elimination: Dexketoprofen Trometamol Case Study
- PMID: 35273497
- PMCID: PMC8904038
- DOI: 10.3389/fphar.2022.808456
A Physiologically Based Pharmacokinetic Model for Studying the Biowaiver Risk of Biopharmaceutics Classification System Class I Drugs With Rapid Elimination: Dexketoprofen Trometamol Case Study
Abstract
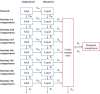
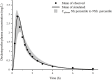
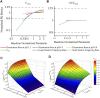
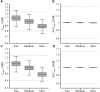
Biowaiver based on the biopharmaceutics classification system (BCS) has been widely used in the global market for the approval of new generic drug products to avoid unnecessary in vivo bioequivalence (BE) studies. However, it is reported that three out of four formulations of dexketoprofen trometamol (DEX) tablets (BCS class I drug) failed the first BE study. The aim of this study was to determine whether the current biowaiver standard is reasonable for DEX. Thus, we successfully established a physiologically based pharmacokinetic (PBPK) model for DEX and examined the effects of dissolution, permeability, and gastric emptying time on DEX absorption under BCS-based biowaiver conditions using sensitivity analyses. Parameter sensitivity analysis showed that the dissolution rate in pH 1.2 media, permeability, and liquid gastric emptying time were sensitive parameters of Cmax. Therefore, gastric emptying variation was introduced into the PBPK model, and virtual BE studys were conducted on original research formulation and the formulation of the boundary dissolution rate (f2 = 50) prescribed by the biowaiver guideline. The virtual BE results showed dissolution rate changes within the biowaiver range will not cause high non-BE ratio, indicate waive of DEX generic drugs would not lead the risk of Cmax when generic products satisfy the requirements of biowaiver guideline. However, the effect of excipients on gastric emptying as a sensitive factor needs to be further studied when the rapid elimination of BCS class I drug is biowaived.
Keywords: bioequivalence; biowaiver; dexketoprofen trometamol; gastric emptying; physiologically based pharmacokinetic model.
Copyright © 2022 Zhang, Ye, Hu, Li, Li, Xiao, Chen and Yang.
Conflict of interest statement
QX was employed by the Mosim Pharmaceutical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

