Proteolytic Cleavage of the Extracellular Domain Affects Signaling of Parathyroid Hormone 1 Receptor
- PMID: 35273573
- PMCID: PMC8902639
- DOI: 10.3389/fendo.2022.839351
Proteolytic Cleavage of the Extracellular Domain Affects Signaling of Parathyroid Hormone 1 Receptor
Abstract
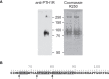
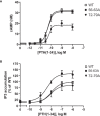
Parathyroid hormone 1 receptor (PTH1R) is a member of the class B family of G protein-coupled receptors, which are characterized by a large extracellular domain required for ligand binding. We have previously shown that the extracellular domain of PTH1R is subject to metalloproteinase cleavage in vivo that is regulated by ligand-induced receptor trafficking and leads to impaired stability of PTH1R. In this work, we localize the cleavage site in the first loop of the extracellular domain using amino-terminal protein sequencing of purified receptor and by mutagenesis studies. We further show, that a receptor mutant not susceptible to proteolytic cleavage exhibits reduced signaling to Gs and increased activation of Gq compared to wild-type PTH1R. These findings indicate that the extracellular domain modulates PTH1R signaling specificity, and that its cleavage affects receptor signaling.
Keywords: GPCRs; biased signaling; ectodomain cleavage; matrix metalloproteinase; parathyroid hormone 1 receptor.
Copyright © 2022 Klenk, Hommers and Lohse.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Jüppner H, Abou-Samra AB, Uneno S, Gu WX, Potts JT, Segre GV. The Parathyroid Hormone-Like Peptide Associated With Humoral Hypercalcemia of Malignancy and Parathyroid Hormone Bind to the Same Receptor on the Plasma Membrane of ROS 17/2.8 Cells. J Biol Chem (1988) 263:8557–60. doi: 10.1016/S0021-9258(18)68339-5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

