Tumor Microenvironment in Acute Myeloid Leukemia: Adjusting Niches
- PMID: 35273598
- PMCID: PMC8901718
- DOI: 10.3389/fimmu.2022.811144
Tumor Microenvironment in Acute Myeloid Leukemia: Adjusting Niches
Abstract
Acute myeloid leukemias (AML) comprise a wide array of different entities, which have in common a rapid expansion of myeloid blast cells leading to displacement of normal hematopoietic cells and also disruption of the microenvironment in the bone marrow niches. Based on an insight into the complex cellular interactions in the bone marrow niches in non-neoplastic conditions in general, this review delineates the complex relationship between leukemic cells and reactive cells of the tumor microenvironment (TME) in AML. A special focus is directed on niche cells and various T-cell subsets as these also provide a potential therapeutic rationale considering e.g. immunomodulation. The TME of AML on the one hand plays a vital role for sustaining and promoting leukemogenesis but - on the other hand - it also has adverse effects on abnormal blasts developing into overt leukemia hindering their proliferation and potentially removing such cells. Thus, leukemic cells need to and develop strategies in order to manipulate the TME. Interference with those strategies might be of particular therapeutic potential since mechanisms of resistance related to tumor cell plasticity do not apply to it.
Keywords: AML; T-cells; TME; bone marrow niches; bone marrow stromal cells.
Copyright © 2022 Menter and Tzankov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
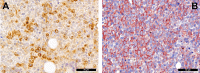
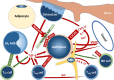
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

