Amelioration of Lupus Serum-Induced Skin Inflammation in CD64-Deficient Mice
- PMID: 35273604
- PMCID: PMC8901504
- DOI: 10.3389/fimmu.2022.824008
Amelioration of Lupus Serum-Induced Skin Inflammation in CD64-Deficient Mice
Abstract
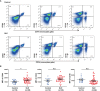
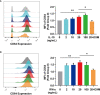
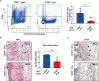
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disorder characterized by high autoantibodies levels and multiorgan tissue damage. The current study investigated the role of CD64 in SLE patients and animal models. According to a flow cytometry study, SLE patients showed an increase in CD64 expression in circulating monocytes. There was a correlation between CD64 and SLEDAI, blood urea nitrogen levels, and anti-Sm antibodies. In skin lesions of lupus MRL/lpr mice, there was high IgG deposition and CD64 expression. In vitro, cytokines IL-10 and IFN-γ upregulated CD64 expression in monocytes/macrophages that was inhibited by glucocorticoids. In CD64-deficient mice, skin inflammation induced by lupus serum was reduced. Furthermore, activation of spleen tyrosine kinase (Syk), Akt, and extracellular signal-regulated kinase (Erk) was inhibited in CD64-deficient monocytes. The results suggest that CD64 could be a biomarker for observing SLE progression, as well as a mechanistic checkpoint in lupus pathogenesis.
Keywords: CD64; flow cytometry; inflammation; monocytes/macrophages; systemic lupus erythematosus.
Copyright © 2022 Jiang, Han, Qiu, Yu, Feng, Wang, Duan and Deng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

