Preclinical experimental models for assessing laxative activities of substances/products under investigation: a scoping review of the literature
- PMID: 35273679
- PMCID: PMC8902583
Preclinical experimental models for assessing laxative activities of substances/products under investigation: a scoping review of the literature
Abstract
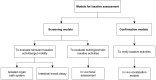
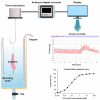
Constipation is a common gastrointestinal problem worldwide. Its impact on health can range from an unpleasant problem to being seriously troublesome. When lifestyle modification fails to deal with constipation, laxatives are the mainstay of therapy. There are several types of laxatives currently available; however, there still remains a need for better laxatives because certain currently available laxatives are not appropriate for or accessible to some patients. Preclinical experiments to study the laxative potential of substances/products of interest are vital to improving that situation. The selection of appropriate experimental models for assessing the laxative activities of substances/products under investigation is crucial to achieving valid and meaningful results. This article provides a scoping review of the literature, outlining, and summarizing models currently being used in preclinical experiments assessing the laxative activities of substances/products under investigation. The review includes both screening models, e.g., the isolated organ bath system, in vivo fecal assessment and intestinal transit assay, and confirmation models, e.g., in vivo constipation models. Chemical substances/drugs used to induce constipation in in vivo constipation models, e.g., loperamide, diphenoxylate, montmorillonite, and clonidine, as well as standard laxative agents used as a positive control in experimental models, e.g., bisacodyl, carbachol, lactulose, sodium picosulfate, castor oil, phenolphthalein, and yohimbine, are described in detail. The purpose of this article is to assist researchers in the design and implementation of preclinical experimental models for assessing laxative activities of substances/products under investigation to achieve valid and meaningful preclinical results prior to experimentation in humans.
Keywords: Laxatives; constipation; drug screening; preclinical drug evaluations.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures
References
-
- Diaz S, Bittar K, Mendez MD. Treasure Island (FL): 2021. Constipation.
-
- Sharma A, Rao S. Constipation: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 2017;239:59–74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources