Safety and Efficacy of a MEURI Program for the Use of High Dose Ivermectin in COVID-19 Patients
- PMID: 35273939
- PMCID: PMC8902036
- DOI: 10.3389/fpubh.2022.813378
Safety and Efficacy of a MEURI Program for the Use of High Dose Ivermectin in COVID-19 Patients
Abstract
Background: In the absence of antiviral alternatives, interventions under research for COVID-19 might be offered following guidelines from WHO for monitored emergency use of unregistered and experimental interventions (MEURI). Ivermectin is among several drugs explored for its role against SARS-CoV-2, with a well-known safety profile but conflicting data regarding clinical utility for COVID-19. The aim of this report is to inform on the results of a MEURI Program of high-dose ivermectin in COVID-19 carried out by the Ministry of Health of the Province of La Pampa, Argentina.
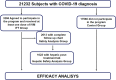
Methods: COVID-19 subjects, within 5 days of symptoms onset were invited to participate in the program, which consisted in the administration of ivermectin 0.6 mg/kg/day for 5 days plus standard of care. Active pharmacosurveillance was performed for 21 days, and hepatic laboratory assessments were performed in a subset of patients. Frequency of Intensive Care Unit (ICU) admission and COVID-19-related mortality of subjects in the ivermectin intention to treat group were compared with that observed in inhabitants of the same province during the same period not participating in the program.
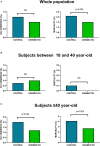
Results: From 21,232 subjects with COVID-19, 3,266 were offered and agreed to participate in the ivermectin program and 17,966 did not and were considered as controls. A total of 567 participants reported 819 adverse events (AEs); 3.13% discontinued ivermectin due to adverse events. ICU admission was significantly lower in the ivermectin group compared to controls among participants ≥40 year-old (1.2 vs. 2.0%, odds ratio 0.608; p = 0.024). Similarly, mortality was lower in the ivermectin group in the full group analysis (1.5 vs. 2.1%, odds ratio 0.720; p = 0.029), as well as in subjects ≥ 40 year- old (2.7 vs. 4.1%, odds ratio 0.655; p = 0.005).
Conclusions: This report highlights the safety and possible efficacy of high dose ivermectin as a potentially useful intervention deserving public health-based consideration for COVID-19 patients.
Keywords: COVID-19; ICU-admission; ivermectin; mortality; safety.
Copyright © 2022 Mayer, Krolewiecki, Ferrero, Bocchio, Barbero, Miguel, Paladini, Delgado, Ojeda, Elorza, Bertone, Fleitas, Vera and Kohan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Johns Hopkins University . COVID-19 Dashboard. Available online at: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/6f1ed23... (accessed January 26, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

