The Cardiac Dysfunction Caused by Metabolic Alterations in Alzheimer's Disease
- PMID: 35274014
- PMCID: PMC8902161
- DOI: 10.3389/fcvm.2022.850538
The Cardiac Dysfunction Caused by Metabolic Alterations in Alzheimer's Disease
Erratum in
-
Corrigendum: The cardiac dysfunction caused by metabolic alterations in Alzheimer's disease.Front Cardiovasc Med. 2025 Aug 8;12:1578594. doi: 10.3389/fcvm.2025.1578594. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40860367 Free PMC article.
Abstract
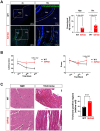
A progressive defect in the energy generation pathway is implicated in multiple aging-related diseases, including cardiovascular conditions and Alzheimer's Disease (AD). However, evidence of the pathogenesis of cardiac dysfunction in AD and the associations between the two organ diseases need further elucidation. This study aims to characterize cellular defects resulting in decreased cardiac function in AD-model. 5XFAD mice, a strain expressing five mutations in human APP and PS1 that shows robust Aβ production with visible plaques at 2 months and were used in this study as a model of AD. 5XFAD mice and wild-type (WT) counterparts were subjected to echocardiography at 2-, 4-, and 6-month, and 5XFAD had a significant reduction in cardiac fractional shortening and ejection fraction compared to WT. Additionally, 5XFAD mice had decreased observed electrical signals demonstrated as decreased R, P, T wave amplitudes. In isolated cardiomyocytes, 5XFAD mice showed decreased fraction shortening, rate of shortening, as well as the degree of transient calcium influx. To reveal the mechanism by which AD leads to cardiac systolic dysfunction, the immunoblotting analysis showed increased activation of AMP-activated protein kinase (AMPK) in 5XFAD left ventricular and brain tissue, indicating altered energy metabolism. Mito Stress Assays examining mitochondrial function revealed decreased basal and maximal oxygen consumption rate, as well as defective pyruvate dehydrogenase activity in the 5XFAD heart and brain. Cellular inflammation was provoked in the 5XFAD heart and brain marked by the increase of reactive oxygen species accumulation and upregulation of inflammatory mediator activities. Finally, AD pathological phenotype with increased deposition of Aβ and defective cognitive function was observed in 6-month 5XFAD mice. In addition, elevated fibrosis was observed in the 6-month 5XFAD heart. The results implicated that AD led to defective mitochondrial function, and increased inflammation which caused the decrease in contractility of the heart.
Keywords: Alzheimer's Disease; cardiac dysfunction; glucose metabolic alterations; metabolic regulation; mitochondrial deficits.
Copyright © 2022 Murphy, Le, Fedorova, Yang, Krause-Hauch, Davitt, Zoungrana, Fatmi, Lesnefsky, Li and Ren.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

