Piezo1 activation attenuates thrombin-induced blebbing in breast cancer cells
- PMID: 35274124
- PMCID: PMC9016622
- DOI: 10.1242/jcs.258809
Piezo1 activation attenuates thrombin-induced blebbing in breast cancer cells
Abstract
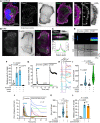
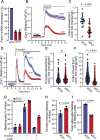
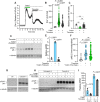
Cancer cells exploit a variety of migration modes to leave primary tumors and establish metastases, including amoeboid cell migration, which is typically reliant on bleb formation. Here we demonstrate that thrombin induces dynamic blebbing in the MDA-MB-231 breast cancer cell line and confirm that protease-activated receptor 1 (PAR1) activation is sufficient to induce this effect. Cell confinement has been implicated as a driving force in bleb-based migration. Unexpectedly, we found that gentle contact compression, exerted using a custom built 'cell press' to mechanically stimulate cells, reduced thrombin-induced blebbing. Thrombin-induced blebbing was similarly attenuated using the small molecule Yoda1, an agonist of the mechanosensitive Ca2+ channel Piezo1, and this attenuation was impaired in Piezo1-depleted cells. Additionally, Piezo1 activation suppressed thrombin-induced phosphorylation of ezrin, radixin and moesin (ERM) proteins, which are implicated in the blebbing process. Our results provide mechanistic insights into Piezo1 activation as a suppressor of dynamic blebbing, specifically that which is induced by thrombin.
Keywords: Blebbing; Breast cancer; Piezo1; Thrombin.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Adyshev, D. M., Dudek, S. M., Moldobaeva, N., Kim, K.-M., Ma, S.-F., Kasa, A., Garcia, J. G. N. and Verin, A. D. (2013). Ezrin/radixin/moesin proteins differentially regulate endothelial hyperpermeability after thrombin. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L240-L255. 10.1152/ajplung.00355.2012 - DOI - PMC - PubMed
-
- Agarwal, P., Lee, H.-P., Smeriglio, P., Grandi, F., Goodman, S., Chaudhuri, O. and Bhutani, N. (2021). A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat. Biomed. Eng. 5, 1472-1484. 10.1038/s41551-021-00691-3 - DOI - PMC - PubMed
-
- Ben-Aissa, K., Patino-Lopez, G., Belkina, N. V., Maniti, O., Rosales, T., Hao, J.-J., Kruhlak, M. J., Knutson, J. R., Picart, C. and Shaw, S. (2012). Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker. J. Biol. Chem. 287, 16311-16323. 10.1074/jbc.M111.304881 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

