Antibodies against biotin-labeled red blood cells can shorten posttransfusion survival
- PMID: 35274303
- PMCID: PMC9007277
- DOI: 10.1111/trf.16849
Antibodies against biotin-labeled red blood cells can shorten posttransfusion survival
Abstract
Background: In hematologic and transfusion medicine research, measurement of red blood cell (RBC) in vivo kinetics must be safe and accurate. Recent reports indicate use of biotin-labeled RBC (BioRBC) to determine red cell survival (RCS) offers substantial advantages over 51 Cr and other labeling methods. Occasional induction of BioRBC antibodies has been reported.
Study design and methods: To investigate the causes and consequences of BioRBC immunization, we reexposed three previously immunized adults to BioRBC and evaluated the safety, antibody emergence, and RCS of BioRBC.
Results: BioRBC re-exposure caused an anamnestic increase of plasma BioRBC antibodies at 5-7 days; all were subclass IgG1 and neutralized by biotinylated albumin, thus indicating structural specificity for the biotin epitope. Concurrently, specific antibody binding to BioRBC was observed in each subject. As biotin label density increased, the proportion of BioRBC that bound increased antibody also increased; the latter was associated with proportional accelerated removal of BioRBC labeled at density 6 μg/mL. In contrast, only one of three subjects exhibited accelerated removal of BioRBC density 2 μg/mL. No adverse clinical or laboratory events were observed. Among three control subjects who did not develop BioRBC antibodies following initial BioRBC exposure, re-exposure induced neither antibody emergence nor accelerated BioRBC removal.
Discussion: We conclude re-exposure of immunized subjects to BioRBC can induce anamnestic antibody response that can cause an underestimation of RCS. To minimize chances of antibody induction and underestimation of RCS, we recommend an initial BioRBC exposure volume of ≤10 mL and label densities of ≤18 μg/mL.
Keywords: BioRBC antibodies; RBC posttransfusion kinetics; biotin; biotin-labeled RBC.
© 2022 AABB.
Conflict of interest statement
CONFLICT OF INTEREST
DMM served as a previous consultant for MedDay Pharmaceuticals, Paris, France, and serves as a current consultant for Nature’s Bounty, Ronkonkoma, NY. AKN North and Melissa von Goetz are employed by Cerus Corporation, 1220 Concord Avenue, Concord, CA 94520. There are no conflicts of interest for the remaining authors.
Figures





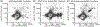

References
-
- Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109–14. - PubMed
-
- Mock DM, Nalbant D, Kyosseva SV, Schmidt RL, An G, Matthews NI, et al. Development, validation, and potential applications of biotinylated red blood cells for posttransfusion kinetics and other physiological studies: evidenced-based analysis and recommendations. Transfusion. 2018;58:2068–81. - PMC - PubMed
-
- Stowell SR, North AK, Franco RS, Cancelas JA, Mock DM, Strauss RG, Geisen C, Schmidt RL, Nalbant D, Widness JA. Shortened survival of biotin-labeled red blood cells (BioRBCs) following a second BioRBC transfusion in an adult with a previous BioRBC antibody response. Transfusion. 2014;54:52A. https://onlinelibrary.wiley.com/doi/10.1111/trf.12845. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL046925/HL/NHLBI NIH HHS/United States
- UL1 TR000039/TR/NCATS NIH HHS/United States
- RR027219/NH/NIH HHS/United States
- U54 TR001629/TR/NCATS NIH HHS/United States
- VA/VA/United States
- UL1TR000039/U54TR001629/UL1TR003107/NH/NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- R01 DK063088 (RSF)/NH/NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- S10 RR027219/RR/NCRR NIH HHS/United States
- R01 DK063088/DK/NIDDK NIH HHS/United States
- R25 HL108825/HL/NHLBI NIH HHS/United States
- U54TR001356 (U Iowa)/NH/NIH HHS/United States
- U54 TR001356/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

