Midsagittal corpus callosal thickness and cognitive impairment in Parkinson's disease
- PMID: 35274408
- PMCID: PMC9314988
- DOI: 10.1111/ejn.15640
Midsagittal corpus callosal thickness and cognitive impairment in Parkinson's disease
Abstract
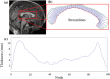
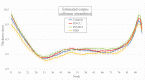
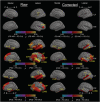
People diagnosed with Parkinson's disease (PD) can experience significant neuropsychiatric symptoms, including cognitive impairment and dementia, the neuroanatomical substrates of which are not fully characterised. Symptoms associated with cognitive impairment and dementia in PD may relate to direct structural changes to the corpus callosum via primary white matter pathology or as a secondary outcome due to the degeneration of cortical regions. Using magnetic resonance imaging, the corpus callosum can be investigated at the midsagittal plane, where it converges to a contiguous mass and is not intertwined with other tracts. The objective of this project was thus twofold: First, we investigated possible changes in the thickness of the midsagittal callosum and cortex in patients with PD with varying levels of cognitive impairment; and secondly, we investigated the relationship between the thickness of the midsagittal corpus callosum and the thickness of the cortex. Study participants included cognitively unimpaired PD participants (n = 35), PD participants with mild cognitive impairment (n = 22), PD participants with dementia (n = 17) and healthy controls (n = 27). We found thinning of the callosum in PD-related dementia compared with PD-related mild cognitive impairment and cognitively unimpaired PD participants. Regression analyses found thickness of the left medial orbitofrontal cortex to be positively correlated with thickness of the anterior callosum in PD-related mild cognitive impairment. This study suggests that a midsagittal thickness model can uncover changes to the corpus callosum in PD-related dementia, which occur in line with changes to the cortex in this advanced disease stage.
Keywords: Parkinson's disease; corpus callosum; dementia; mild cognitive impairment.
© 2022 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no competing interests.
Figures



Similar articles
-
White matter abnormalities in the corpus callosum with cognitive impairment in Parkinson disease.Neurology. 2018 Dec 11;91(24):e2244-e2255. doi: 10.1212/WNL.0000000000006646. Epub 2018 Nov 14. Neurology. 2018. PMID: 30429273 Free PMC article.
-
Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease.Neurology. 2017 Mar 28;88(13):1265-1272. doi: 10.1212/WNL.0000000000003764. Epub 2017 Feb 24. Neurology. 2017. PMID: 28235816 Free PMC article.
-
Tracking Cortical Changes Throughout Cognitive Decline in Parkinson's Disease.Mov Disord. 2020 Nov;35(11):1987-1998. doi: 10.1002/mds.28228. Epub 2020 Sep 4. Mov Disord. 2020. PMID: 32886420
-
Neural Correlates of Cognitive Impairment in Parkinson's Disease: A Review of Structural MRI Findings.Int Rev Neurobiol. 2019;144:1-28. doi: 10.1016/bs.irn.2018.09.009. Epub 2018 Oct 16. Int Rev Neurobiol. 2019. PMID: 30638452 Review.
-
Corpus callosum atrophy is a possible indicator of region- and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis.Arch Neurol. 1998 Feb;55(2):193-8. doi: 10.1001/archneur.55.2.193. Arch Neurol. 1998. PMID: 9482361 Review.
Cited by
-
Morphological basis of Parkinson disease-associated cognitive impairment: an update.J Neural Transm (Vienna). 2022 Aug;129(8):977-999. doi: 10.1007/s00702-022-02522-4. Epub 2022 Jun 20. J Neural Transm (Vienna). 2022. PMID: 35726096 Review.
-
Pathobiology of Cognitive Impairment in Parkinson Disease: Challenges and Outlooks.Int J Mol Sci. 2023 Dec 29;25(1):498. doi: 10.3390/ijms25010498. Int J Mol Sci. 2023. PMID: 38203667 Free PMC article. Review.
-
Effect of glucocorticoid therapy on brain white matter microstructure in thyroid-associated ophthalmopathy: a longitudinal diffusion kurtosis imaging study.Brain Imaging Behav. 2025 Aug;19(4):919-929. doi: 10.1007/s11682-025-01025-6. Epub 2025 Jun 1. Brain Imaging Behav. 2025. PMID: 40450654
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

