Inhibition of MicroRNA-92a Improved Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats via Suppressing Oxidative Stress and Endothelial Dysfunction
- PMID: 35274504
- PMCID: PMC9826907
- DOI: 10.5534/wjmh.210177
Inhibition of MicroRNA-92a Improved Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats via Suppressing Oxidative Stress and Endothelial Dysfunction
Abstract
Purpose: To determine whether microRNA could be a therapy target of erectile dysfunction (ED) and the underlying mechanisms.
Materials and methods: Eight-week-old fasting male SD rats were intraperitoneally injected with streptozotocin to construct diabetic rat models. Diabetic ED rats were treated with miRNA-92a inhibitor. The cavernous nerves were electrically stimulated to measure the intracavernous pressure and mean arterial pressure of rats in each group. After the detection, the penile cavernous tissues are properly stored for subsequent experiments. Rat aortic endothelial cells were used in in vitro studies.
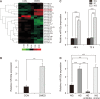
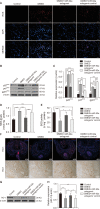
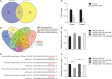
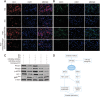
Results: The expression of miR-92a was significantly increased in the corpus cavernosum of Streptozocin (STZ)-induced diabetic rats and injection of miR-92a antagomir into the corpus cavernosum of diabetic rats significantly increased eNOS/NO/cGMP signaling pathway activities, cavernous endothelial cell proliferation, endothelial cell-cell junction protein expression and decreased the levels of oxidative stress. These changes restored erectile function in STZ-induced diabetic rats. Moreover, in vitro study demonstrated that the miR-92a expression increased significantly in endothelial cells treated with high glucose, inhibiting AMPK/eNOS and AMPK/Nrf2/HO-1 signaling pathways in rat aortic endothelial cells via targeting Prkaa2, causing endothelial dysfunction and overactive oxidative stress, miR-92a inhibitor can improve the above parameters.
Conclusions: miRNA-92a inhibitor could exert an inhibition role on oxidative stress and endothelial dysfunction to improve diabetic ED effectively.
Keywords: Endothelial cells; Erectile dysfunction; Molecular targeted therapy; Oxidative stress; microRNA.
Copyright © 2023 Korean Society for Sexual Medicine and Andrology.
Conflict of interest statement
The authors have nothing to disclose.
Figures


References
-
- Mitidieri E, Cirino G, d'Emmanuele di Villa Bianca R, Sorrentino R. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. - PubMed
-
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. - PubMed
-
- McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12 Suppl 4:S6–S11. - PubMed
-
- Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129–136. - PubMed
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. - PubMed

