Differentiation of circulating monocytes into macrophages with metabolically activated phenotype regulates inflammation in dyslipidemia patients
- PMID: 35274685
- PMCID: PMC9113394
- DOI: 10.1093/cei/uxac013
Differentiation of circulating monocytes into macrophages with metabolically activated phenotype regulates inflammation in dyslipidemia patients
Abstract
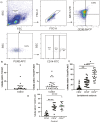
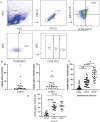
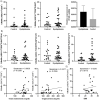
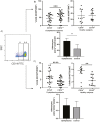
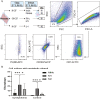
Macrophages are mediators of inflammation having an important role in the pathogenesis of cardiovascular diseases. Recently, a pro-inflammatory subpopulation, known as metabolically activated macrophages (MMe), has been described in conditions of obesity and metabolic syndrome where they are known to release cytokines that can promote insulin resistance. Dyslipidemia represents an important feature in metabolic syndrome and corresponds to one of the main modifiable risk factors for the development of cardiovascular diseases. Circulating monocytes can differentiate into macrophages under certain conditions. They correspond to a heterogeneous population, which include inflammatory and anti-inflammatory subsets; however, there is a wide spectrum of phenotypes. Therefore, we decided to investigate whether the metabolic activated monocyte (MoMe) subpopulation is already present under dyslipidemia conditions. Secondly, we assessed whether different levels of cholesterol and triglycerides play a role in the polarization towards the metabolic phenotype (MMe) of macrophages. Our results indicate that MoMe cells are found in both healthy and dyslipidemia patients, with cells displaying the following metabolic phenotype: CD14varCD36+ABCA1+PLIN2+. Furthermore, the percentages of CD14++CD68+CD80+ pro-inflammatory monocytes are higher in dyslipidemia than in healthy subjects. When analysing macrophage differentiation, we observed that MMe percentages were higher in the dyslipidemia group than in healthy subjects. These MMe have the ability to produce high levels of IL-6 and the anti-inflammatory cytokine IL-10. Furthermore, ABCA1 expression in MMe correlates with LDL serum levels. Our study highlights the dynamic contributions of metabolically activated macrophages in dyslipidemia, which may have a complex participation in low-grade inflammation due to their pro- and anti-inflammatory function.
Keywords: Metabolically activated macrophages; dyslipidemia; immune regulation; interleukin-10 (IL-10); monocytes.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Immunology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-disea...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

