Treatment Patterns, Health Care Resource Utilization, and Cost in Patients with Myelofibrosis in the United States
- PMID: 35274711
- PMCID: PMC8914486
- DOI: 10.1093/oncolo/oyab058
Treatment Patterns, Health Care Resource Utilization, and Cost in Patients with Myelofibrosis in the United States
Abstract
Background: This study analyses treatment patterns, health care resource utilization (HCRU), and costs in patients with myelofibrosis (MF) and a subgroup treated with ruxolitinib (RUX).
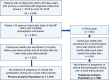
Materials and methods: Treatment patterns, all-cause and MF-related HCRU, and costs were analyzed in adults with MF with continuous enrollment in a commercial or the Medicare Advantage health plan in the pre-index period, defined as the 12 months immediately prior to the index date (date of primary or secondary MF diagnosis), and the post-index period, defined as ≥6 months following the index date. In a subgroup analysis, outcomes were analyzed in patients treated with optimal RUX (OPT RUX, ≥30 mg) and suboptimal RUX (SUB RUX, <30 mg) in the pre-index RUX period, defined as the 3 months immediately prior to the index RUX date (first date for an RUX claim), and the post-index RUX period, defined as ≥6 months following the index RUX date.
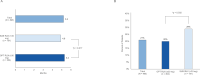
Results: Of 2830 patients with an MF diagnosis, 1191 met eligibility requirements. The median age of patients was 72 years, 54% were male, and comorbidities were frequent. Sixty percent of patients received ≥1 line of therapy (LOT), of which 46% (n = 331) had ≥2 LOTs during the post-index MF period. Costs increased considerably 6-month pre-index to 6-month post-index (all-cause: cause ($24,216 to $48,966) and MF-related ($16,502 to $39,383), driven by inpatient stays and pharmacy costs. In the subgroup analysis, patients treated with RUX (n = 495) experienced significant disease burden and high costs, regardless of dose. A shorter duration of therapy and a higher rate of discontinuation were observed in patients treated with SUB RUX (n = 191) versus OPT RUX (n = 304).
Conclusion: These findings suggest a significant disease and economic impacts associated with MF patients that persists with RUX therapy, highlighting the need for additional therapeutic options for MF.
Keywords: burden; costs; health care resource utilization; myelofibrosis; ruxolitinib.
© The Author(s) 2022. Published by Oxford University Press.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

