An Evolutionarily Conserved AU-Rich Element in the 3' Untranslated Region of a Transcript Misannotated as a Long Noncoding RNA Regulates RNA Stability
- PMID: 35274990
- PMCID: PMC9022526
- DOI: 10.1128/mcb.00505-21
An Evolutionarily Conserved AU-Rich Element in the 3' Untranslated Region of a Transcript Misannotated as a Long Noncoding RNA Regulates RNA Stability
Abstract
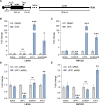
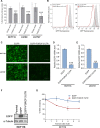
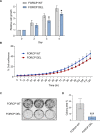
One of the primary mechanisms of post-transcriptional gene regulation is the modulation of RNA stability. We recently discovered that LINC00675, a transcript annotated as a long noncoding RNA (lncRNA), is transcriptionally regulated by FOXA1 and encodes a highly conserved small protein that localizes to the endoplasmic reticulum, hence renamed as FORCP (FOXA1-regulated conserved small protein). Here, we show that the endogenous FORCP transcript is rapidly degraded and rendered unstable as a result of 3'UTR-mediated degradation. Surprisingly, although the FORCP transcript is a canonical nonsense-mediated decay (NMD) and microRNA (miRNA) target, we found that it is not degraded by NMD or miRNAs. Targeted deletion of an evolutionarily conserved region in the FORCP 3'UTR using CRISPR/Cas9 significantly increased the stability of the FORCP transcript. Interestingly, this region requires the presence of an immediate downstream 55-nt-long sequence for transcript stability regulation. Functionally, colorectal cancer cells lacking this conserved region expressed from the endogenous FORCP locus displayed decreased proliferation and clonogenicity. These data demonstrate that the FORCP transcript is destabilized via conserved elements within its 3'UTR and emphasize the need to interrogate the function of a given 3'UTR in its native context.
Keywords: 3’UTR; ARE; AU-rich; CRISPR/Cas9; FORCP; FOXA1; LINC00675; NMD; RNA stability; TMEM238L; conserved; lincRNA; lncRNA; mRNA decay; mRNA stability; miRNA; micropeptide; misannotated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A small protein encoded by a putative lncRNA regulates apoptosis and tumorigenicity in human colorectal cancer cells.Elife. 2020 Oct 28;9:e53734. doi: 10.7554/eLife.53734. Elife. 2020. PMID: 33112233 Free PMC article.
-
A natural "GA" insertion mutation in the sequence encoding the 3'UTR of CXCL12/SDF-1α: Identification, characterization, and functional impact on mRNA splicing.Gene. 2019 Jan 10;681:36-44. doi: 10.1016/j.gene.2018.09.045. Epub 2018 Sep 28. Gene. 2019. PMID: 30266500
-
Oncogenic p95HER2 regulates Na+-HCO3- cotransporter NBCn1 mRNA stability in breast cancer cells via 3'UTR-dependent processes.Biochem J. 2016 Nov 1;473(21):4027-4044. doi: 10.1042/BCJ20160054. Epub 2016 Sep 8. Biochem J. 2016. PMID: 27609814
-
The Role of 3'UTR of RNA Viruses on mRNA Stability and Translation Enhancement.Mini Rev Med Chem. 2021;21(16):2389-2398. doi: 10.2174/1389557521666210217092305. Mini Rev Med Chem. 2021. PMID: 33596798 Review.
-
Implications of polyadenylation in health and disease.Nucleus. 2014;5(6):508-19. doi: 10.4161/nucl.36360. Epub 2014 Oct 31. Nucleus. 2014. PMID: 25484187 Free PMC article. Review.
Cited by
-
Micropeptides: origins, identification, and potential role in metabolism-related diseases.J Zhejiang Univ Sci B. 2023 Dec 15;24(12):1106-1122. doi: 10.1631/jzus.B2300128. J Zhejiang Univ Sci B. 2023. PMID: 38057268 Free PMC article. Review.
-
TET2 regulation of alcoholic fatty liver via Srebp1 mRNA in paraspeckles.iScience. 2024 Feb 20;27(3):109278. doi: 10.1016/j.isci.2024.109278. eCollection 2024 Mar 15. iScience. 2024. PMID: 38482502 Free PMC article.
-
Staufen1 Represses the FOXA1-Regulated Transcriptome by Destabilizing FOXA1 mRNA in Colorectal Cancer Cells.Mol Cell Biol. 2024;44(2):43-56. doi: 10.1080/10985549.2024.2307574. Epub 2024 Feb 12. Mol Cell Biol. 2024. PMID: 38347726 Free PMC article.
References
-
- Garcia-Cardenas JM, Guerrero S, Lopez-Cortes A, Armendariz-Castillo I, Guevara-Ramirez P, Perez-Villa A, Yumiceba V, Zambrano AK, Leone PE, Paz YMC. 2019. Post-transcriptional regulation of colorectal cancer: a focus on RNA-binding proteins. Front Mol Biosci 6:65. 10.3389/fmolb.2019.00065. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
