Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review
- PMID: 35275085
- PMCID: PMC8956992
- DOI: 10.2196/34410
Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review
Abstract
Background: HIV assays designed to detect recent infection, also known as "recency assays," are often used to estimate HIV incidence in a specific country, region, or subpopulation, alone or as part of recent infection testing algorithms (RITAs). Recently, many countries and organizations have become interested in using recency assays within case surveillance systems and routine HIV testing services to measure other indicators beyond incidence, generally referred to as "non-incidence surveillance use cases."
Objective: This review aims to identify published evidence that can be used to validate methodological approaches to recency-based incidence estimation and non-incidence use cases. The evidence identified through this review will be used in the forthcoming technical guidance by the World Health Organization (WHO) and United Nations Programme on HIV/AIDS (UNAIDS) on the use of HIV recency assays for identification of epidemic trends, whether for HIV incidence estimation or non-incidence indicators of recency.
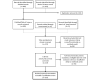
Methods: To identify the best methodological and field implementation practices for the use of recency assays to estimate HIV incidence and trends in recent infections for specific populations or geographic areas, we conducted a systematic review of the literature to (1) understand the use of recency testing for surveillance in programmatic and laboratory settings, (2) review methodologies for implementing recency testing for both incidence estimation and non-incidence use cases, and (3) assess the field performance characteristics of commercially available recency assays.
Results: Among the 167 documents included in the final review, 91 (54.5%) focused on assay or algorithm performance or methodological descriptions, with high-quality evidence of accurate age- and sex-disaggregated HIV incidence estimation at national or regional levels in general population settings, but not at finer geographic levels for prevention prioritization. The remaining 76 (45.5%) described the field use of incidence assays including field-derived incidence (n=45), non-incidence (n=25), and both incidence and non-incidence use cases (n=6). The field use of incidence assays included integrating RITAs into routine surveillance and assisting with molecular genetic analyses, but evidence was generally weaker or only reported on what was done, without validation data or findings related to effectiveness of using non-incidence indicators calculated through the use of recency assays as a proxy for HIV incidence.
Conclusions: HIV recency assays have been widely validated for estimating HIV incidence in age- and sex-specific populations at national and subnational regional levels; however, there is a lack of evidence validating the accuracy and effectiveness of using recency assays to identify epidemic trends in non-incidence surveillance use cases. More research is needed to validate the use of recency assays within HIV testing services, to ensure findings can be accurately interpreted to guide prioritization of public health programming.
Keywords: HIV; incidence; recency; recent infection; surveillance.
©Shelley N Facente, Eduard Grebe, Andrew D Maher, Douglas Fox, Susan Scheer, Mary Mahy, Shona Dalal, David Lowrance, Kimberly Marsh. Originally published in JMIR Public Health and Surveillance (https://publichealth.jmir.org), 11.03.2022.
Conflict of interest statement
Conflicts of Interest: SNF and EG have received consulting income and research support from Sedia Biosciences Corporation, Gilead Sciences, and through the CDC-funded TRACE program, as a subcontract from the University of California, San Francisco.
Figures

References
-
- UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance . WHO. Geneva: 2011. [2022-01-21]. When and How to Use Assays for Recent Infection to Estimate HIV Incidence at a Population Level. https://www.who.int/diagnostics_laboratory/hiv_incidence_may13_final.pdf .
-
- World Health Organization . Geneva: World Health Organization; 2009. Oct 05, [2022-01-22]. Meeting Report: WHO Technical Working Group on HIV Incidence Assays (Cape Town, South Africa, 16 and 17 July) https://www.who.int/diagnostics_laboratory/links/hiviwg_capetown_07_09.pdf .
-
- Welte A, McWalter T A, Laeyendecker O, Hallett T B. Using tests for recent infection to estimate incidence: problems and prospects for HIV. Euro Surveill. 2010 Jun 17;15(24) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19589 - PMC - PubMed
-
- Kassanjee Reshma, McWalter Thomas A, Bärnighausen Till, Welte Alex. A new general biomarker-based incidence estimator. Epidemiology. 2012 Sep;23(5):721–8. doi: 10.1097/EDE.0b013e3182576c07. http://europepmc.org/abstract/MED/22627902 - DOI - PMC - PubMed
-
- FIND Target Product Profile for Tests for Recent HIV Infection. Find. 2017. Feb, [2022-01-21]. https://www.finddx.org/wp-content/uploads/2019/03/HIV-Incidence-TPP-FIND... .
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

