Safety and Efficacy of Monoclonal Antibodies for Alzheimer's Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials
- PMID: 35275549
- PMCID: PMC9198746
- DOI: 10.3233/JAD-220046
Safety and Efficacy of Monoclonal Antibodies for Alzheimer's Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials
Abstract
Background: Monoclonal antibodies (mAbs) are currently among the most investigated targets for potential disease-modifying therapies in Alzheimer's disease (AD).
Objective: Our objectives were to identify all registered trials investigating mAbs in MCI due to AD or AD at any stage, retrieve available published and unpublished data from all registered trials, and analyze data on safety and efficacy outcomes.
Methods: A systematic search of all registered trials on ClinicalTrials.gov and EUCT was performed. Available results were searched on both platforms and on PubMed, ISI Web of Knowledge, and The Cochrane Library.
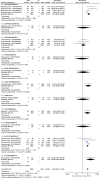
Results: Overall, 101 studies were identified on 27 mAbs. Results were available for 50 trials investigating 12 mAbs. For 18 trials, data were available from both published and unpublished sources, for 21 trials only from published sources, and for 11 trials only from unpublished sources. Meta-analyses of amyloid-related imaging abnormalities (ARIA) events showed overall risk ratios of 10.65 for ARIA-E and of 1.75 for ARIA-H. The meta-analysis of PET-SUVR showed an overall significant effect of mAbs in reducing amyloid (SMD -0.88), but when considering clinical efficacy, data on CDR-SB showed that treated patients had a statistically significant but clinically non-relevant lower worsening (MD -0.15).
Conclusion: Our results suggest that the risk-benefit profile of mAbs remains unclear. Research should focus on clarifying the effect of amyloid on cognitive decline, providing data on treatment response rate, and accounting for minimal clinically important difference. Research on mAbs should also investigate the possible long-term impact of ARIA events, including potential factors predicting their onset.
Keywords: Alzheimer’s disease; amyloid; meta-analysis; mild cognitive impairment; monoclonal antibodies; safety; systematic review; treatment outcome.
Conflict of interest statement
Authors’ disclosures available online (
Figures




References
-
- Tian Hui Kwan A, Arfaie S, Therriault J, Rosa-Neto P, Gauthier S (2020) lessons learnt from the second generation of anti-amyloid monoclonal antibodies clinical trials. Dement Geriatr Cogn Disord 49, 334–348. - PubMed
-
- Food & Drug Administration. FDA news release. FDA Grants Accelerated Approval for Alzheimer’s Drug. Available at: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat.... Last updated June 7, 2021, Accessed on February 9, 2022
-
- Planche V, Villain N (2021) US Food and Drug Administration approval of Aducanumab-Is amyloid load a valid surrogate end point for Alzheimer disease clinical trials? JAMA Neurol 78, 1307–1308. - PubMed
-
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K (2019) Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol 76, 915–924. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

