The Use and Duration of Preintubation Respiratory Support Is Associated With Increased Mortality in Immunocompromised Children With Acute Respiratory Failure
- PMID: 35275593
- PMCID: PMC9707852
- DOI: 10.1097/CCM.0000000000005535
The Use and Duration of Preintubation Respiratory Support Is Associated With Increased Mortality in Immunocompromised Children With Acute Respiratory Failure
Abstract
Objectives: To determine the association between preintubation respiratory support and outcomes in patients with acute respiratory failure and to determine the impact of immunocompromised (IC) diagnoses on outcomes after adjustment for illness severity.
Design: Retrospective multicenter cohort study.
Setting: Eighty-two centers in the Virtual Pediatric Systems database.
Patients: Children 1 month to 17 years old intubated in the PICU who received invasive mechanical ventilation (IMV) for greater than or equal to 24 hours.
Interventions: None.
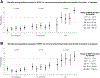
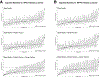
Measurements and main results: High-flow nasal cannula (HFNC) or noninvasive positive-pressure ventilation (NIPPV) or both were used prior to intubation in 1,825 (34%) of 5,348 PICU intubations across 82 centers. When stratified by IC status, 50% of patients had no IC diagnosis, whereas 41% were IC without prior hematopoietic cell transplant (HCT) and 9% had prior HCT. Compared with patients intubated without prior support, preintubation exposure to HFNC (adjusted odds ratio [aOR], 1.33; 95% CI, 1.10-1.62) or NIPPV (aOR, 1.44; 95% CI, 1.20-1.74) was associated with increased odds of PICU mortality. Within subgroups of IC status, preintubation respiratory support was associated with increased odds of PICU mortality in IC patients (HFNC: aOR, 1.50; 95% CI, 1.11-2.03; NIPPV: aOR, 1.76; 95% CI, 1.31-2.35) and HCT patients (HFNC: aOR, 1.75; 95% CI, 1.07-2.86; NIPPV: aOR, 1.85; 95% CI, 1.12-3.02) compared with IC/HCT patients intubated without prior respiratory support. Preintubation exposure to HFNC/NIPPV was not associated with mortality in patients without an IC diagnosis. Duration of HFNC/NIPPV greater than 6 hours was associated with increased mortality in IC HCT patients (HFNC: aOR, 2.41; 95% CI, 1.05-5.55; NIPPV: aOR, 2.53; 95% CI, 1.04-6.15) and patients compared HCT patients with less than 6-hour HFNC/NIPPV exposure. After adjustment for patient and center characteristics, both preintubation HFNC/NIPPV use (median, 15%; range, 0-63%) and PICU mortality varied by center.
Conclusions: In IC pediatric patients, preintubation exposure to HFNC and/or NIPPV is associated with increased odds of PICU mortality, independent of illness severity. Longer duration of exposure to HFNC/NIPPV prior to IMV is associated with increased mortality in HCT patients.
Copyright © 2022 by the Society of Critical Care Medicine and Wolters Kluwer Health, Inc. All Rights Reserved.
Conflict of interest statement
Dr. Lindell is supported by the Thrasher Research Fund. Dr. Fitzgerald is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK119463. Dr. Rowan is supported by the National Heart, Lung and Blood Institute (NHLBI) K23HL150244. Dr. Flori is supported by NHLBI R01HL149910 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) R21HD097387. Dr. Napolitano is supported by Agency for Healthcare Research and Quality (AHRQ) R18HS024511 and has research or consulting relationships with Drager, Smiths Medical, Philips/Respironics, Actuated Medical, and VERO-Biotech. Dr. Nishisaki is supported by the AHRQ R18 HS024511. Drs. Fitzgerald, Rowan, and Flori received support for article research from the National Institutes of Health (NIH). Dr. Rowan’s institution received funding from the NHLBI (K23HL150244). Dr. Flori’s institution received funding from the Society of Critical Care Medicine; she disclosed that she is an unpaid advisor to Aerogen Pharma, an unpaid member of Executive Board for Michigan Medical Society, and an unpaid member of Executive Committee for Pediatric Acute Lung Injury and Sepsis Investigators Network. Dr. Nishisaki’s institution received funding from the AHRQ (R18HS024511 and R03HS026939); he received support for article research from the AHRQ. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
Should We Intubate Pediatric Hematopoietic Cell Transplant Patients With Respiratory Failure Sooner?Crit Care Med. 2022 Jul 1;50(7):1163-1167. doi: 10.1097/CCM.0000000000005548. Epub 2022 Jun 13. Crit Care Med. 2022. PMID: 35726983 Free PMC article. No abstract available.
References
-
- Munoz-Bonet JI, Flor-Macian EM, Brines J, Rosello-Millet PM, et al.: Predictive factors for the outcome of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 2010; 11(6):675–680 - PubMed
-
- Fortenberry JD, Del Toro J, Jefferson LS, Evey L, et al.: Management of pediatric acute hypoxemic respiratory insufficiency with bilevel positive pressure (BiPAP) nasal mask ventilation. Chest 1995; 108(4):1059–1064 - PubMed
-
- Baudin F, Gagnon S, Crulli B, Proulx F, et al.: Modalities and Complications Associated With the Use of High-Flow Nasal Cannula: Experience in a Pediatric ICU. Respir Care 2016; 61(10):1305–1310 - PubMed
-
- Teague WG: Noninvasive ventilation in the pediatric intensive care unit for children with acute respiratory failure. Pediatr Pulmonol 2003; 35(6):418–426 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

