Rigidified Derivative of the Non-macrocyclic Ligand H4OCTAPA for Stable Lanthanide(III) Complexation
- PMID: 35275621
- PMCID: PMC8965877
- DOI: 10.1021/acs.inorgchem.2c00501
Rigidified Derivative of the Non-macrocyclic Ligand H4OCTAPA for Stable Lanthanide(III) Complexation
Abstract
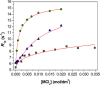
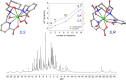
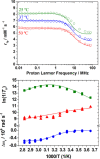
The stability constants of lanthanide complexes with the potentially octadentate ligand CHXOCTAPA4-, which contains a rigid 1,2-diaminocyclohexane scaffold functionalized with two acetate and two picolinate pendant arms, reveal the formation of stable complexes [log KLaL = 17.82(1) and log KYbL = 19.65(1)]. Luminescence studies on the Eu3+ and Tb3+ analogues evidenced rather high emission quantum yields of 3.4 and 11%, respectively. The emission lifetimes recorded in H2O and D2O solutions indicate the presence of a water molecule coordinated to the metal ion. 1H nuclear magnetic relaxation dispersion profiles and 17O NMR chemical shift and relaxation measurements point to a rather low water exchange rate of the coordinated water molecule (kex298 = 1.58 × 106 s-1) and relatively high relaxivities of 5.6 and 4.5 mM-1 s-1 at 20 MHz and 25 and 37 °C, respectively. Density functional theory calculations and analysis of the paramagnetic shifts induced by Yb3+ indicate that the complexes adopt an unprecedented cis geometry with the two picolinate groups situated on the same side of the coordination sphere. Dissociation kinetics experiments were conducted by investigating the exchange reactions of LuL occurring with Cu2+. The results confirmed the beneficial effect of the rigid cyclohexyl group on the inertness of the Lu3+ complex. Complex dissociation occurs following proton- and metal-assisted pathways. The latter is relatively efficient at neutral pH, thanks to the formation of a heterodinuclear hydroxo complex.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Lanthanide dota-like complexes containing a picolinate pendant: structural entry for the design of Ln(III)-based luminescent probes.Inorg Chem. 2011 May 2;50(9):4125-41. doi: 10.1021/ic2001915. Epub 2011 Apr 1. Inorg Chem. 2011. PMID: 21456610
-
Lanthanide(III) complexes with a reinforced cyclam ligand show unprecedented kinetic inertness.J Am Chem Soc. 2014 Dec 31;136(52):17954-7. doi: 10.1021/ja511331n. Epub 2014 Dec 19. J Am Chem Soc. 2014. PMID: 25495928
-
H4octapa: highly stable complexation of lanthanide(III) ions and copper(II).Inorg Chem. 2015 Mar 2;54(5):2345-56. doi: 10.1021/ic502966m. Epub 2015 Feb 18. Inorg Chem. 2015. PMID: 25692564
-
Lanthanide complexes based on a diazapyridinophane platform containing picolinate pendants.Inorg Chem. 2012 Oct 15;51(20):10893-903. doi: 10.1021/ic301369z. Epub 2012 Sep 27. Inorg Chem. 2012. PMID: 23016509
-
Stability, water exchange, and anion binding studies on lanthanide(III) complexes with a macrocyclic ligand based on 1,7-diaza-12-crown-4: extremely fast water exchange on the Gd3+ complex.Inorg Chem. 2009 Sep 21;48(18):8878-89. doi: 10.1021/ic9011197. Inorg Chem. 2009. PMID: 19655713
Cited by
-
Gd(III) and Yb(III) Complexes Derived from a New Water-Soluble Dioxopolyazacyclohexane Macrocycle.ACS Omega. 2023 Sep 11;8(38):34575-34582. doi: 10.1021/acsomega.3c03454. eCollection 2023 Sep 26. ACS Omega. 2023. PMID: 37779985 Free PMC article.
-
Effect of Magnetic Anisotropy on the 1H NMR Paramagnetic Shifts and Relaxation Rates of Small Dysprosium(III) Complexes.Inorg Chem. 2023 Sep 4;62(35):14326-14338. doi: 10.1021/acs.inorgchem.3c01959. Epub 2023 Aug 21. Inorg Chem. 2023. PMID: 37602400 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

