Predicting future community-level ocular Chlamydia trachomatis infection prevalence using serological, clinical, molecular, and geospatial data
- PMID: 35275911
- PMCID: PMC8942265
- DOI: 10.1371/journal.pntd.0010273
Predicting future community-level ocular Chlamydia trachomatis infection prevalence using serological, clinical, molecular, and geospatial data
Abstract
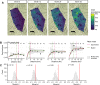
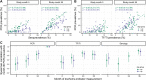
Trachoma is an infectious disease characterized by repeated exposures to Chlamydia trachomatis (Ct) that may ultimately lead to blindness. Efficient identification of communities with high infection burden could help target more intensive control efforts. We hypothesized that IgG seroprevalence in combination with geospatial layers, machine learning, and model-based geostatistics would be able to accurately predict future community-level ocular Ct infections detected by PCR. We used measurements from 40 communities in the hyperendemic Amhara region of Ethiopia to assess this hypothesis. Median Ct infection prevalence among children 0-5 years old increased from 6% at enrollment, in the context of recent mass drug administration (MDA), to 29% by month 36, following three years without MDA. At baseline, correlation between seroprevalence and Ct infection was stronger among children 0-5 years old (ρ = 0.77) than children 6-9 years old (ρ = 0.48), and stronger than the correlation between active trachoma and Ct infection (0-5y ρ = 0.56; 6-9y ρ = 0.40). Seroprevalence was the strongest concurrent predictor of infection prevalence at month 36 among children 0-5 years old (cross-validated R2 = 0.75, 95% CI: 0.58-0.85), though predictive performance declined substantially with increasing temporal lag between predictor and outcome measurements. Geospatial variables, a spatial Gaussian process, and stacked ensemble machine learning did not meaningfully improve predictions. Serological markers among children 0-5 years old may be an objective tool for identifying communities with high levels of ocular Ct infections, but accurate, future prediction in the context of changing transmission remains an open challenge.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. The Lancet. 2014. Dec 13;384(9960):2142–52. - PubMed
-
- World Health Organization. WHO Alliance for the Global Elimination of Trachoma by 2020: progress report, 2019. World Health Organization; 2020 Jul p. 349–60. (Weekly epidemiological record). Report No.: 30.
-
- World Health Organization. Validation of elimination of trachoma as a public health problem [Internet]. Geneva: World Health Organization; 2016. [cited 2021 Apr 6]. Report No.: WHO/ HTM/NTD/2016.8. Available from: https://apps.who.int/iris/bitstream/handle/10665/208901/WHO-HTM-NTD-2016...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

