Cost-effectiveness of Fecal Microbiota Transplantation for First Recurrent Clostridioides difficile Infection
- PMID: 35275989
- PMCID: PMC9617579
- DOI: 10.1093/cid/ciac207
Cost-effectiveness of Fecal Microbiota Transplantation for First Recurrent Clostridioides difficile Infection
Abstract
Background: Both the American College of Gastroenterology and the Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America 2021 Clostridioides difficile infection (CDI) guidelines recommend fecal microbiota transplantation (FMT) for persons with multiple recurrent CDI. Emerging data suggest that FMT may have high cure rates when used for first recurrent CDI. The aim of this study was to assess the cost-effectiveness of FMT for first recurrent CDI.
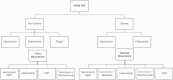
Methods: We developed a Markov model to simulate a cohort of patients presenting with initial CDI infection. The model estimated the costs, effectiveness, and cost-effectiveness of different CDI treatment regimens recommended in the 2021 IDSA guidelines, with the additional option of FMT for first recurrent CDI. The model includes stratification by the severity of initial infection, estimates of cure, recurrence, and mortality. Data sources were taken from IDSA guidelines and published literature on treatment outcomes. Outcome measures were quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs).
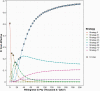
Results: When FMT is available for first recurrent CDI, the optimal cost-effective treatment strategy is fidaxomicin for initial nonsevere CDI, vancomycin for initial severe CDI, and FMT for first and subsequent recurrent CDI, with an ICER of $27 135/QALY. In probabilistic sensitivity analysis at a $100 000 cost-effectiveness threshold, FMT for first and subsequent CDI recurrence was cost-effective 90% of the time given parameter uncertainty.
Conclusions: FMT is a cost-effective strategy for first recurrent CDI. Prospective evaluation of FMT for first recurrent CDI is warranted to determine the efficacy and risk of recurrence.
Keywords: Clostridium difficile infection; cost-effectiveness; fecal microbiota transplantation.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. E. A. E. reports grants from the National Institutes of Health, during the conduct of the study, and grants or contracts from the Minnesota Department of Health and Minnesota Department of Human Services and personal fees from ViiV Healthcare and Janssen Pharmaceuticals, outside the submitted work. B. P. V. reports consulting fees for Prometheus and grants from Takeda, Roche, Celgene, Diasorin, and Genentech, outside the submitted work. R. R. reports a grant from the National Institutes of Allergy and Infectious Diseases (K23AI13885). E. S. A. reports no potential conflicts.
Figures
Similar articles
-
Cost-effectiveness of Treatment Regimens for Clostridioides difficile Infection: An Evaluation of the 2018 Infectious Diseases Society of America Guidelines.Clin Infect Dis. 2020 Feb 14;70(5):754-762. doi: 10.1093/cid/ciz318. Clin Infect Dis. 2020. PMID: 31001619 Free PMC article.
-
Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis.Clin Infect Dis. 2014 Jun;58(11):1507-14. doi: 10.1093/cid/ciu128. Epub 2014 Mar 31. Clin Infect Dis. 2014. PMID: 24692533 Free PMC article.
-
Cost-effectiveness analysis of sequential fecal microbiota transplantation for fulminant Clostridioides difficile infection.J Gastroenterol Hepatol. 2021 Sep;36(9):2432-2440. doi: 10.1111/jgh.15483. Epub 2021 Mar 17. J Gastroenterol Hepatol. 2021. PMID: 33682170
-
Navigating changes in Clostridioides difficile prevention and treatment.J Manag Care Spec Pharm. 2020 Dec;26(12-a Suppl):S3-S23. doi: 10.18553/jmcp.2020.26.12-a.s3. J Manag Care Spec Pharm. 2020. PMID: 33533699 Free PMC article. Review.
-
Systematic Review with Meta-Analysis: Fecal Microbiota Transplantation for Severe or Fulminant Clostridioides difficile.Dig Dis Sci. 2022 Mar;67(3):978-988. doi: 10.1007/s10620-021-06908-4. Epub 2021 Mar 22. Dig Dis Sci. 2022. PMID: 33748913
Cited by
-
Clostridioides difficile infection: history, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options.Clin Microbiol Rev. 2024 Jun 13;37(2):e0013523. doi: 10.1128/cmr.00135-23. Epub 2024 Feb 29. Clin Microbiol Rev. 2024. PMID: 38421181 Free PMC article. Review.
-
The Role of Akkermansia muciniphila in Disease Regulation.Probiotics Antimicrob Proteins. 2025 Jul 9. doi: 10.1007/s12602-025-10642-y. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40632459 Review.
-
Fidaxomicin Use in the Pediatric Population with Clostridioides difficile.Clin Pharmacol. 2022 Sep 23;14:91-98. doi: 10.2147/CPAA.S273318. eCollection 2022. Clin Pharmacol. 2022. PMID: 36177387 Free PMC article. Review.
-
Fecal microbiota transplantation for treatment of refractory or recurrent Clostridioides difficile infection in Taiwan: a cost-effectiveness analysis.Front Med (Lausanne). 2023 Oct 2;10:1229148. doi: 10.3389/fmed.2023.1229148. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37849493 Free PMC article.
-
Cost-Effectiveness Analysis of REBYOTA™ (Fecal Microbiota, Live-jslm [FMBL]) Versus Standard of Care for the Prevention of Recurrent Clostridioides difficile Infection in the USA.Adv Ther. 2023 Jun;40(6):2784-2800. doi: 10.1007/s12325-023-02505-1. Epub 2023 Apr 24. Adv Ther. 2023. PMID: 37093359 Free PMC article.
References
-
- McFarland LV, Elmer GW, Surawicz CM.. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. - PubMed
-
- Mcfarland LV, Surawicz CM, Elmer GW, et al. . A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–8. - PubMed

