Genetic Diagnostics in Routine Osteological Assessment of Adult Low Bone Mass Disorders
- PMID: 35276006
- PMCID: PMC9202726
- DOI: 10.1210/clinem/dgac147
Genetic Diagnostics in Routine Osteological Assessment of Adult Low Bone Mass Disorders
Abstract
Context: Many different inherited and acquired conditions can result in premature bone fragility/low bone mass disorders (LBMDs).
Objective: We aimed to elucidate the impact of genetic testing on differential diagnosis of adult LBMDs and at defining clinical criteria for predicting monogenic forms.
Methods: Four clinical centers broadly recruited a cohort of 394 unrelated adult women before menopause and men younger than 55 years with a bone mineral density (BMD) Z-score < -2.0 and/or pathological fractures. After exclusion of secondary causes or unequivocal clinical/biochemical hallmarks of monogenic LBMDs, all participants were genotyped by targeted next-generation sequencing.
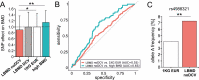
Results: In total, 20.8% of the participants carried rare disease-causing variants (DCVs) in genes known to cause osteogenesis imperfecta (COL1A1, COL1A2), hypophosphatasia (ALPL), and early-onset osteoporosis (LRP5, PLS3, and WNT1). In addition, we identified rare DCVs in ENPP1, LMNA, NOTCH2, and ZNF469. Three individuals had autosomal recessive, 75 autosomal dominant, and 4 X-linked disorders. A total of 9.7% of the participants harbored variants of unknown significance. A regression analysis revealed that the likelihood of detecting a DCV correlated with a positive family history of osteoporosis, peripheral fractures (> 2), and a high normal body mass index (BMI). In contrast, mutation frequencies did not correlate with age, prevalent vertebral fractures, BMD, or biochemical parameters. In individuals without monogenic disease-causing rare variants, common variants predisposing for low BMD (eg, in LRP5) were overrepresented.
Conclusion: The overlapping spectra of monogenic adult LBMD can be easily disentangled by genetic testing and the proposed clinical criteria can help to maximize the diagnostic yield.
Keywords: genetic risk score; genotype-phenotype correlation; low bone mass disorder; monogenic disorder; osteoporosis; rare genetic variant.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol. 2015;11(8):462-474. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

