ENaC activation by proteases
- PMID: 35276025
- PMCID: PMC9540061
- DOI: 10.1111/apha.13811
ENaC activation by proteases
Abstract
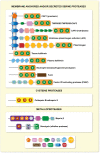
Proteases are fundamental for a plethora of biological processes, including signalling and tissue remodelling, and dysregulated proteolytic activity can result in pathogenesis. In this review, we focus on a subclass of membrane-bound and soluble proteases that are defined as channel-activating proteases (CAPs), since they induce Na+ ion transport through an autocrine mechanism when co-expressed with the highly amiloride-sensitive epithelial sodium channel (ENaC) in Xenopus oocytes. These experiments first identified CAP1 (channel-activating protease 1, prostasin) followed by CAP2 (channel-activating protease 2, TMPRSS4) and CAP3 (channel-activating protease 3, matriptase) as in vitro mediators of ENaC current. Since then, more serine-, cysteine- and metalloproteases were confirmed as in vitro CAPs that potentially cleave and regulate ENaC, and thus this nomenclature was not further followed, but is accepted as functional term or alias. The precise mechanism of ENaC modulation by proteases has not been fully elucidated. Studies in organ-specific protease knockout models revealed evidence for their role in increasing ENaC activity, although the proteases responsible for ENaC activation are yet to be identified. We summarize recent findings in animal models of these CAPs with respect to their implication in ENaC activation. We discuss the consequences of dysregulated CAPs underlying epithelial phenotypes in pathophysiological conditions, and the role of selected protease inhibitors. We believe that these proteases may present interesting therapeutic targets for diseases with aberrant sodium homoeostasis.
Keywords: epithelial phenotype; epithelial sodium channel; homoeostasis; kidney disease.
© 2022 The Authors. Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Vallet V, Chraibi A, Gaeggeler H‐P, Horisberger J‐D, Rossier BC. An epithelial serine protease activates the amiloride‐sensitive sodium channel. Nature. 1997;389(6651):607‐610. - PubMed
-
- Vuagniaux G, Vallet V, Jaeger NF, et al. Activation of the amiloride‐sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11(5):828‐834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

