A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy
- PMID: 35276062
- PMCID: PMC8986623
- DOI: 10.1016/j.cmet.2022.02.009
A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy
Abstract
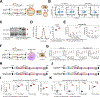
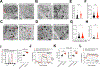
Chimeric antigen receptor (CAR)-T cell-based immunotherapy for cancer and immunological diseases has made great strides, but it still faces multiple hurdles. Finding the right molecular targets to engineer T cells toward a desired function has broad implications for the armamentarium of T cell-centered therapies. Here, we developed a dead-guide RNA (dgRNA)-based CRISPR activation screen in primary CD8+ T cells and identified gain-of-function (GOF) targets for CAR-T engineering. Targeted knockin or overexpression of a lead target, PRODH2, enhanced CAR-T-based killing and in vivo efficacy in multiple cancer models. Transcriptomics and metabolomics in CAR-T cells revealed that augmenting PRODH2 expression reshaped broad and distinct gene expression and metabolic programs. Mitochondrial, metabolic, and immunological analyses showed that PRODH2 engineering enhances the metabolic and immune functions of CAR-T cells against cancer. Together, these findings provide a system for identification of GOF immune boosters and demonstrate PRODH2 as a target to enhance CAR-T efficacy.
Keywords: CAR-T; PRODH2; T cell CRISPR activation screen; T cell GOF screen; antitumor efficacy; dead-guide RNA; metabolism; mitochondria; proline metabolism.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.C. is a cofounder of EvolveImmune Tx and Cellinfinity Bio. A patent has been filed by Yale University related to this study (S.C., L.Y., J.J.P., L.P., R.D.C., and M.B.D. as inventors). The other authors declare no competing interests.
Figures





References
-
- Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA, Delgoffe GM, et al. (2016). Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity 45, 358–373. - PMC - PubMed
-
- Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. - PubMed
-
- Brown CE, and Mackall CL (2019). CAR T cell therapy: inroads to response and resistance. Nat Rev Immunol 19, 73–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

