Integration of vascular progenitors into functional blood vessels represents a distinct mechanism of vascular growth
- PMID: 35276066
- PMCID: PMC9365108
- DOI: 10.1016/j.devcel.2022.02.015
Integration of vascular progenitors into functional blood vessels represents a distinct mechanism of vascular growth
Abstract
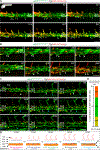
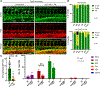
During embryogenesis, the initial vascular network forms by the process of vasculogenesis, or the specification of vascular progenitors de novo. In contrast, the majority of later-forming vessels arise by angiogenesis from the already established vasculature. Here, we show that new vascular progenitors in zebrafish embryos emerge from a distinct site along the yolk extension, or secondary vascular field (SVF), incorporate into the posterior cardinal vein, and contribute to subintestinal vasculature even after blood circulation has been initiated. We further demonstrate that SVF cells participate in vascular recovery after chemical ablation of vascular endothelial cells. Inducible inhibition of the function of vascular progenitor marker etv2/etsrp prevented SVF cell differentiation and resulted in the defective formation of subintestinal vasculature. Similar late-forming etv2+ progenitors were also observed in mouse embryos, suggesting that SVF cells are evolutionarily conserved. Our results characterize a distinct mechanism by which new vascular progenitors incorporate into established vasculature.
Keywords: ER71; Etsrp; Etv2; blood vessel; mouse; progenitors; vascular endothelium; vasculogenesis; zebrafish.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Etv2 and fli1b function together as key regulators of vasculogenesis and angiogenesis.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):865-76. doi: 10.1161/ATVBAHA.114.304768. Epub 2015 Feb 26. Arterioscler Thromb Vasc Biol. 2015. PMID: 25722433 Free PMC article.
-
Vegf signaling promotes vascular endothelial differentiation by modulating etv2 expression.Dev Biol. 2017 Apr 15;424(2):147-161. doi: 10.1016/j.ydbio.2017.03.005. Epub 2017 Mar 7. Dev Biol. 2017. PMID: 28279709 Free PMC article.
-
Ets1 functions partially redundantly with Etv2 to promote embryonic vasculogenesis and angiogenesis in zebrafish.Dev Biol. 2020 Sep 1;465(1):11-22. doi: 10.1016/j.ydbio.2020.06.007. Epub 2020 Jul 3. Dev Biol. 2020. PMID: 32628937 Free PMC article.
-
ETS Transcription Factor ETV2/ER71/Etsrp in Hematopoietic and Vascular Development.Curr Top Dev Biol. 2016;118:77-111. doi: 10.1016/bs.ctdb.2016.01.005. Epub 2016 Feb 12. Curr Top Dev Biol. 2016. PMID: 27137655 Review.
-
How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos.Dev Cell. 2017 Sep 25;42(6):567-583. doi: 10.1016/j.devcel.2017.08.015. Dev Cell. 2017. PMID: 28950100 Review.
Cited by
-
Temporally and regionally distinct morphogenetic processes govern zebrafish caudal fin blood vessel network expansion.Development. 2023 Apr 1;150(7):dev201030. doi: 10.1242/dev.201030. Epub 2023 Apr 11. Development. 2023. PMID: 36938965 Free PMC article.
-
Systemic brain dissemination of glioblastoma requires transdifferentiation into endothelial-like cells via TGF-β-ALK1-Smad1/5 signaling.Neoplasia. 2025 Feb;60:101110. doi: 10.1016/j.neo.2024.101110. Epub 2024 Dec 25. Neoplasia. 2025. PMID: 39724753 Free PMC article.
-
Lineage labeling with zebrafish hand2 Cre and CreERT2 recombinase CRISPR knock-ins.bioRxiv [Preprint]. 2024 Dec 5:2024.12.04.626907. doi: 10.1101/2024.12.04.626907. bioRxiv. 2024. Update in: Dev Dyn. 2025 Mar 26. doi: 10.1002/dvdy.70022. PMID: 39677658 Free PMC article. Updated. Preprint.
-
Direct specification of lymphatic endothelium from mesenchymal progenitors.Nat Cardiovasc Res. 2025 Jan;4(1):45-63. doi: 10.1038/s44161-024-00570-5. Epub 2025 Jan 2. Nat Cardiovasc Res. 2025. PMID: 39747454 Free PMC article.
-
Endothelial cell transitions in zebrafish vascular development.Dev Growth Differ. 2024 Aug;66(6):357-368. doi: 10.1111/dgd.12938. Epub 2024 Jul 27. Dev Growth Differ. 2024. PMID: 39072708 Free PMC article. Review.
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, and Isner JM (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science (80-. ). 275, 964–967. - PubMed
-
- Beis D, and Stainier DYR (2006). In vivo cell biology: following the zebrafish trend. Trends Cell Biol. 16, 105–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

