Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons
- PMID: 35276083
- PMCID: PMC9119918
- DOI: 10.1016/j.neuron.2022.02.010
Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons
Abstract
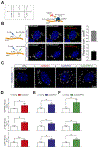
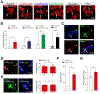
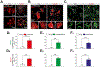
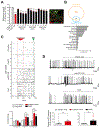
Non-cell-autonomous mechanisms contribute to neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), in which astrocytes release unidentified factors that are toxic to motoneurons (MNs). We report here that mouse and patient iPSC-derived astrocytes with diverse ALS/FTD-linked mutations (SOD1, TARDBP, and C9ORF72) display elevated levels of intracellular inorganic polyphosphate (polyP), a ubiquitous, negatively charged biopolymer. PolyP levels are also increased in astrocyte-conditioned media (ACM) from ALS/FTD astrocytes. ACM-mediated MN death is prevented by degrading or neutralizing polyP in ALS/FTD astrocytes or ACM. Studies further reveal that postmortem familial and sporadic ALS spinal cord sections display enriched polyP staining signals and that ALS cerebrospinal fluid (CSF) exhibits increased polyP concentrations. Our in vitro results establish excessive astrocyte-derived polyP as a critical factor in non-cell-autonomous MN degeneration and a potential therapeutic target for ALS/FTD. The CSF data indicate that polyP might serve as a new biomarker for ALS/FTD.
Keywords: ALS; C9ORF72; CSF; FTD; SOD1; TARDBP; astrocytes; iPSCs; motor neurons; polyP.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that based on the polyP data presented here, we are in the process of filing for a diagnosis and treatment patent application (PCT).
Figures

Comment in
-
Why you always in a mood? Pumpin' polyP, actin' brand new.Neuron. 2022 May 18;110(10):1603-1605. doi: 10.1016/j.neuron.2022.04.003. Neuron. 2022. PMID: 35588711
References
-
- Andreeva N, Ledova L, Ryazanova L, Tomashevsky A, Kulakovskaya T, and Eldarov M (2019). Ppn2 endopolyphosphatase overexpressed in Saccharomyces cerevisiae: Comparison with Ppn1, Ppx1, and Ddp1 polyphosphatases. Biochimie 163, 101–107. - PubMed
-
- Angelova PR, Agrawalla BK, Elustondo PA, Gordon J, Shiba T, Abramov AY, Chang Y-T, and Pavlov EV (2014). In Situ Investigation of Mammalian Inorganic Polyphosphate Localization Using Novel Selective Fluorescent Probes JC-D7 and JC-D8. ACS Chem. Biol 9, 2101–2110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

