Making Statistical Sense of the Molnupiravir MOVe-OUT Clinical Trial
- PMID: 35276667
- PMCID: PMC9128711
- DOI: 10.4269/ajtmh.21-1339
Making Statistical Sense of the Molnupiravir MOVe-OUT Clinical Trial
Abstract
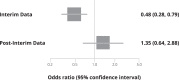
Oral therapies for the early treatment of COVID-19 may prevent disease progression and health system overcrowding. A new oral therapeutic named molnupiravir has been promoted as providing an approximately 50% reduction in death or the need for hospitalization. The clinical trial evaluating this drug was stopped early at the recommendation of the Data Safety and Monitoring Board after approximately 50% of the sample had been recruited. At the point of discontinuing the trial, approximately 90% of the planned sample had been recruited and had available follow-up data accessible. We discuss issues about the study conduct, analysis, and interpretation, including 1) the authors and sponsors presented the interim analysis as the primary analysis; 2) communication between sponsors and the Data Safety and Monitoring Board was insufficient; 3) the treatment effects reverse when examining only the post-interim analysis population, and are substantially attenuated when examining the full data; 4) the choice of primary analysis is incorrect; 5) analysis of lost-to-follow-up patients favors the study drug; and 6) other known molnupiravir trials were not presented in the primary study findings. As a result of methodological and statistical concerns, it seems that external trials, separate from those supported by the sponsoring company, are required to determine the utility of this drug.
Figures
References
-
- Merck News Release , 2021. Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available at: https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-u....
-
- Lakens D , 2020. P-hacking and Optional Stopping Have Been Judged Violations of Scientific Integrity. Available at: http://daniellakens.blogspot.com/2020/09/p-hacking-and-optional-stopping.... Accessed September 29, 2020.
LinkOut - more resources
Full Text Sources
Research Materials