Co-toxicity of Endotoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis
- PMID: 35276782
- PMCID: PMC8840142
- DOI: 10.3390/nu14030424
Co-toxicity of Endotoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis
Abstract
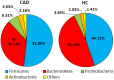
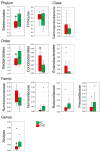
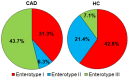
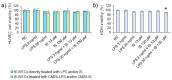
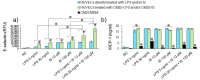
Gut dysbiosis, alongside a high-fat diet and cigarette smoking, is considered one of the factors promoting coronary arterial disease (CAD) development. The present study aimed to research whether gut dysbiosis can increase bacterial metabolites concentration in the blood of CAD patients and what impact these metabolites can exert on endothelial cells. The gut microbiomes of 15 age-matched CAD patients and healthy controls were analyzed by 16S rRNA sequencing analysis. The in vitro impact of LPS and indoxyl sulfate at concentrations present in patients' sera on endothelial cells was investigated. 16S rRNA sequencing analysis revealed gut dysbiosis in CAD patients, further confirmed by elevated LPS and indoxyl sulfate levels in patients' sera. CAD was associated with depletion of Bacteroidetes and Alistipes. LPS and indoxyl sulfate demonstrated co-toxicity to endothelial cells inducing reactive oxygen species, E-selectin, and monocyte chemoattractant protein-1 (MCP-1) production. Moreover, both of these metabolites promoted thrombogenicity of endothelial cells confirmed by monocyte adherence. The co-toxicity of LPS and indoxyl sulfate was associated with harmful effects on endothelial cells, strongly suggesting that gut dysbiosis-associated increased intestinal permeability can initiate or promote endothelial inflammation and atherosclerosis progression.
Keywords: Bacteroidetes; LPS; coronary artery disease; dysbiosis; gut microbiome; indoxyl sulfate; obesity.
Conflict of interest statement
The authors declare no known competing financial interests or personal relationships that could influence the work reported in this paper.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

